Aorto-left ventricular tunnel is a relatively uncommon congenital cardiac anomaly initially documented by Björk and Crafoord in 1947. Reference Bjork and Crafoord1 It manifests as an anomalous communication between the ascending aorta and the left ventricle, bypassing the aortic valve. This abnormal connection leads to the shunting of oxygenated blood from the aorta into the left ventricle, potentially resulting in a spectrum of clinical symptoms and complications. Reference McKay2 This case report explores the necessity of early surgical intervention in aorto-left ventricular tunnel.
Case report
A 26-year-old primigravida woman with hearing impairment delivered a male baby weighing 2970 m at 39 weeks of gestation through vaginal delivery without the need for a postnatal incubator. At 2 months of age, the baby was brought to our hospital due to complaints of fatigue during breastfeeding. Physical examination revealed a body weight of 4600 m and a length of 57 cm, with no dysmorphic features. A 2/6 systolic-diastolic murmur was auscultated, and a posterior-anterior chest X-ray revealed cardiomegaly. Echocardiography disclosed a mildly enlarged left ventricle with dilated sinuses of Valsalva (17 mm, Z score + 3.97). During diastole, an abnormal aorta-left ventricle tunnel-like appearance was observed, originating from the proximal left coronary cusp of the aortic valve and extending into the left ventricle, with a flow velocity of 2 m/s. Left ventricle fractional shortening was 41%, and trabecular thickening was noted in the posterior left ventricle wall. The aortic valve was tricuspid, and no evidence of atrioventricular valve insufficiency was observed (Fig. 1a, b). Although conventional angiography was performed to support transthoracic echocardiography, it was not very successful in showing aorto-left ventricular tunnel. As a matter of fact, cMRI was not very successful in the same way and transthoracic echocardiography was shared in our article as a more demonstrative imaging. Urgent surgical intervention was planned. Under balanced general anaesthesia with Near-Infrared Spectroscopy (NIRS) monitoring (INVOS™ System, Medtronic, MN, USA), a total thymectomy was performed through a median sternotomy. A 2 x 2 cm sinus of Valsalva aneurysm was observed medial to the right coronary ostium in the ascending aortic root. Aorto-bicaval cannulation was performed, and cardiopulmonary bypass was initiated. At 32°C, the aorta was cross-clamped, and antegrade cold blood cardioplegia was delivered while pressure was applied over the tunnel from outside. Subsequently, a transverse partial aortotomy was performed, and an additional dose of cardioplegia was administered to the right coronary artery. According to the Hovaguiman classification, a type II aorto-left ventricular tunnel was observed, appearing as a tunnel with a 2 x 1.5 cm wide passage leading to the left ventricle, identified behind the left coronary cusp. Reference Wong, Amelia, Mohd Zain and Sayuti3 The aortotomy was advanced towards the aneurysm within the tunnel, and the ventricular entry of the tunnel was clearly visualised (Fig. 2). The portion of the tunnel opening into the ventricle was closed using continuous 6.0 polypropylene sutures with a porcine pericardial patch. The entry of the tunnel on the aortic side, located at the upper border of the left coronary cusp, was also closed with the edge of the same patch. The opened aneurysmatic area was closed using a continuous technique. The aortotomy was closed with double rows of 6.0 polypropylene sutures. Following de-airing, the cross-clamp was removed, and the heart resumed spontaneous sinus rhythm. Cardiopulmonary bypass was discontinued without the need for positive inotropic support. The patient’s post-operative period in the ICU was uneventful, and the patient was discharged from the hospital without complications after 5 days. During the 6-month follow-up, physical examination and echocardiography revealed normal findings, with no signs of heart failure.

Figure 1. a : Transthoracic echocardiography apical three-chamber view in diastole displaying abnormal retrograde flow from the aorta to the left ventricle (arrow). b : Transthoracic parasternal long-axis echocardiography in diastole revealing abnormal retrograde flow from the aorta to the left ventricle (arrow).
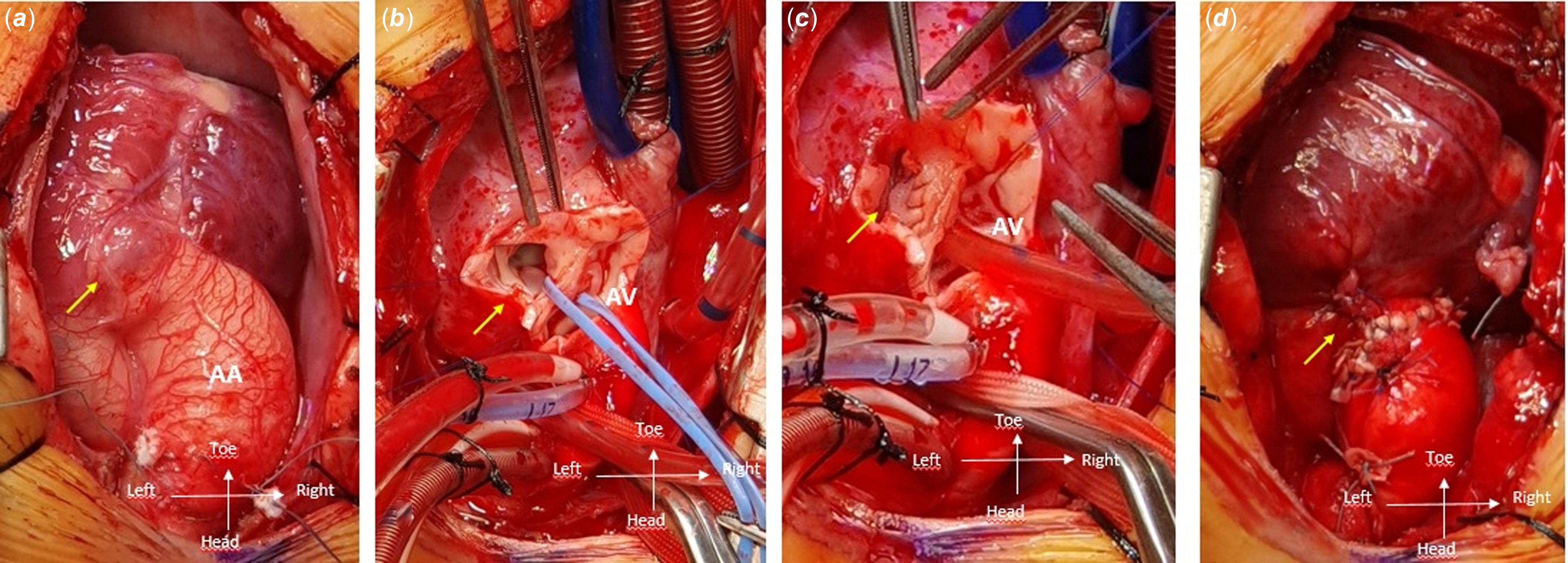
Figure 2. a : Intraoperative images presenting the external view of the aorto-left ventricular tunnel (aorto-left ventricular tunnel, arrow). b : Incision made above the aorto-left ventricular tunnel (arrow) after aortotomy. c : Closure of the aorto-left ventricular tunnel using a single pericardial patch (arrow). d : Final view of the aorto-left ventricular tunnel repair (arrow), with labels for the ascending aorta (AA) and aortic valve (AV).
Discussion
Aorto-left ventricular tunnel can present in various ways, with symptoms in the infantile period often dependent on the size and location of the tunnel. Reference Wong, Amelia, Mohd Zain and Sayuti3 Infants and children with large tunnels may develop congestive heart failure, exhibiting signs such as tachypnoea, poor feeding, and failure to thrive. Some patients may remain asymptomatic and reach the second decade of life without developing heart failure. Reference Bjork and Crafoord1–Reference Wong, Amelia, Mohd Zain and Sayuti3
The diagnosis of aorto-left ventricular tunnel typically involves a combination of clinical evaluation, non-invasive imaging techniques, and cardiac catheterisation. Prenatal diagnosis can be made with fetal echocardiography after 18 weeks of gestation. Reference Chowdhury, Anderson and George4 Aortic left ventricular tunnels originating from the left coronary sinus can be challenging to diagnose and may be mistaken for aortic root dilation and ascending aortic aneurysms. While echocardiography can facilitate diagnosis, other imaging modalities may also be necessary. Reference Yildirim, Erek, Uslu, Saygili and Karaagac5 Differential diagnoses include aortic regurgitation, Valsalva fistula, common arterial trunk, aortopulmonary window, and coronary ventricular fistula. Echocardiography, cardiac MRI, or CT scans might be required. Reference Staniczek, Michalczyk, Stojko and Wloch6 Therefore, we employed all these modalities to ensure accurate diagnosis. While conventional angiography was employed to complement transthoracic echocardiography, it proved less efficacious in delineating aorto-left ventricular tunnel. Similarly, cMRI did not demonstrate significant success in this regard. In our article, transthoracic echocardiography is advocated as a more demonstrative imaging modality.
The management of aorto-left ventricular tunnel hinges on several factors, including the patient’s age, symptoms, and the size and location of the tunnel. Staniczek et al. suggested that early diagnosis and intervention are associated with more favourable outcomes, with many patients achieving normal cardiac function following successful tunnel closure. Reference Staniczek, Michalczyk, Stojko and Wloch6,Reference Sathe, Tomar and Krasemann7 Our patient underwent surgery promptly upon presentation, and the intervention occurred before the left ventricular functions deteriorated.
Mueller et al. described a technique that approaches aorto-left ventricular tunnel through a transverse aortotomy and a transverse right ventricular infundibulotomy. They create an incision in the left lateral free wall of the tunnel, providing access for surgical correction. Reference Mueller, Dave and Prêtre8 In contrast, we preferred advancing the aortotomy towards the aneurysm within the tunnel, enabling clear visualisation of the ventricular entry of the tunnel. This approach facilitated the closure of the tunnel’s entrance from the aortic side and the exit from the ventricular side using a single patch. Long-term follow-up is essential to monitor for potential residual shunts, valve regurgitation, or other complications.
While surgical closure has traditionally been the gold standard, transcatheter techniques have emerged as a promising alternative. Ongoing research and interdisciplinary collaboration in the field of paediatric cardiology will contribute to a better understanding of aorto-left ventricular tunnel and the refinement of therapeutic strategies, ultimately enhancing the quality of life for individuals affected by this condition. In asymptomatic or older children, transcatheter closure using occluder devices has emerged as a less invasive alternative, especially for smaller tunnels. Reference Sathe, Tomar and Krasemann7 Long-term follow-up is crucial to monitor for potential residual shunts, valve regurgitation, or other complications.
Conclusion
This case report suggests that early surgical intervention may be beneficial for patients with aorto-left ventricular tunnel, including asymptomatic individuals. Surgical intervention can effectively mitigate the risk of heart failure and associated complications. In neonates and infants with severe symptoms, early surgical intervention may be necessary. However, it is important to emphasise that this report represents a single-case observation. Further comprehensive studies involving a larger patient cohort are warranted to substantiate the advantages of early surgical management for aorto-left ventricular tunnel.
Acknowledgements
We express our gratitude to Professor Timur MESE for his unwavering support at every stage of this case’s management.
Financial support
The authors declare that this study has received no financial support.
Competing interests
None.
Consent
Written informed consent was obtained from the guardian (parents) of the patient who participated in this case.





