Irritable bowel syndrome is the major cause of referrals to gastroenterology clinics in the Western world, at an estimated cost of $8 billion per annum in the USA (Reference Thompson, Longstreth, Drossman, Drossman, Corazziari and TalleyThompson et al, 2002). Psychiatric disorders occur in 50-60% of these clinic patients and contribute to impaired health-related quality of life and increased medical help-seeking (Reference CreedCreed, 1999; Reference Creed, Ratcliffe and FernandezCreed et al, 2001; American Gastroenterology Association, 2002). It is not clear, however, which psychiatric disorders contribute to these poor outcomes in irritable bowel syndrome and no treatment trial has yet matched type of treatment to specific psychiatric disorders in this population. We have shown that psychodynamic interpersonal therapy and pharmacotherapy with a selective serotonin reuptake inhibitor (paroxetine) each led to improved health-related quality of life in patients with severe irritable bowel syndrome, compared with usual treatment, without additional healthcare costs (Reference Creed, Fernandes and GuthrieCreed et al, 2003). In this study we examined the extent to which particular psychiatric disorders contribute to impairment of role function and high costs at the start of the trial and at follow-up. We predicted that after controlling for severity of abdominal pain, the presence of one or more psychiatric disorders at baseline would be associated with greater impairment in daily activities, reduced ability to work and higher costs. We tested this hypothesis both at baseline (trial entry) and at follow-up (15 months later). We also predicted that the number of psychiatric disorders would be significantly associated with degree of impairment at both trial entry and at follow-up, and that reduction of psychiatric symptoms would be associated with reductions in impairment and costs.
METHOD
We recruited patients from three secondary and four tertiary level gastroenterology clinics around Manchester and Sheffield in the UK. Patients were invited to enter a randomised controlled trial, which involved random allocation to eight sessions of individual psychotherapy over 12 weeks, 20 mg daily of the selective serotonin reuptake inhibitor (SSRI) antidepressant, paroxetine, for 12 weeks or continued routine care by the patient's gastroenterologist and primary care physician (Reference Creed, Fernandes and GuthrieCreed et al, 2003). The same outcome measurements were made at baseline (trial entry) and at follow-up 1 year after the completion of the treatment period (i.e. 15 months after trial entry).
All people attending the clinics with irritable bowel syndrome were screened and those who fulfilled the inclusion criteria were invited to enter the trial. These were patients 18-65 years old who fulfilled the Rome I criteria for this syndrome (Reference Thompson, Creed and DrossmanThompson et al, 1992), with symptoms for 6 months or more and a pain severity score of more than 59 on a 0 to 100 visual analogue scale, who had not responded to ‘usual’ medical treatment including education, dietary advice, and antispasmodic and laxatives or antidiarrhoeal medication. We excluded patients who were unable to complete questionnaires in English, who had a psychotic disorder, severe personality disorder or active suicidal ideation, or had a contraindication either to psychotherapy or to taking an SSRI (e.g. taking medication that could interact with an SSRI, such as sumatriptan, warfarin and anticonvulsants).
Ethics committee approval was obtained for each hospital taking part in the study and all participants signed written consent forms to participate in the study. Before randomisation the trial participants underwent the following assessments.
Assessment of abdominal pain and bowel symptoms
The diagnosis of irritable bowel syndrome according to the Rome criteria was made using the relevant questionnaire, which includes a measure of pain severity, using a visual analogue scale (Reference Drossman, Li and TonerDrossman et al, 1995). Since abdominal pain is the cardinal symptom of the syndrome (Reference Maxton, Morris and WhorwellMaxton et al, 1989; American Gastroenterology Association, 2002), severity of pain was used as the prime measure of severity of the syndrome. Patients also completed a daily diary recording the severity of ten symptoms (nausea, abdominal pain, flatulence, bloating, hard stool, loose stool, urgency, straining, incomplete evacuation and mucus), using a seven-point Likert scale. This diary was kept for 14 days prior to trial entry and follow-up, and the mean daily symptom score was used in the analysis.
Assessment of psychiatric disorder
Psychiatric disorder at trial entry was diagnosed according to ICD-10 criteria (World Health Organization, 1992) using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN; World Health Organization, 1994), administered by a trained psychiatrist. The details of each psychiatric symptom are recorded directly on to a computerised program that produces psychiatric diagnoses according to standardised research criteria. Nine disorders are considered in this report (see Results). The SCAN produces multiple psychiatric diagnoses and the number of psychiatric diagnoses is also quoted in this report. The Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) was used to measure severity of depression.
Outcomes
Impairment of daily activities
Impairment of daily activities was measured in two ways. First, each participant noted the number of days over the previous month in which normal activities were restricted because of the syndrome. Second, the role limitation (physical) sub-scale of the 36-item Short Form Health Survey (SF-36; Reference Ware and SherbourneWare & Sherborne, 1992) was used. This scale closely captures the most common concerns of patients with irritable bowel syndrome - impairment of work, home life and social activities. It is the SF-36 sub-scale that shows least correlation with neuroticism and with psychological distress (Reference Whitehead, Burnett and CookWhitehead et al, 1996) and the greatest sensitivity to severity of irritable bowel syndrome, most clearly distinguishing patients with the syndrome from normal controls (Reference Hahn, Kirchdoerfer and FullertonHahn et al, 1997).
Healthcare and other costs
All patients received healthcare from a single health provider, the UK National Health Service (NHS), so utilisation data were taken directly from the patient's hospital and primary care notes. Direct healthcare costs were derived by applying an appropriate unit cost to each recorded contact or episode of care. These included hospital in-patient days, out-patient, day-patient and accident and emergency department attendances, and all primary care contacts (medical consultations and home visits, practice nurse and practice-based counsellors). Additional costs were derived from an interview with the patient using the Client Service Receipt Inventory (Reference Beecham, Knapp, Thornicroft, Brewin and WingBeecham & Knapp, 1992); these included use of day centres, alternative therapies and prescribed medications, and indirect costs such as travel costs and additional patient expenditure as a result of the illness, non-prescription medication and any additional expenditure relating to housework, childcare or personal care. Indirect (loss of productivity) costs were measured by applying the patient's daily wage to the number of days lost either through illness or clinic attendance. The total of all healthcare, indirect and productivity costs is quoted in the results.
Data analysis
In preliminary analyses we compared patients with and without each psychiatric disorder (see Table 1) using one-way analysis of variance (ANOVA). Patients with and patients without any psychiatric diagnosis, using the criterion of SCAN Index of Definition (ID) level 5 or above, were compared using χ2 and t-tests (see Table 2). In the main data analysis the predictor variables were psychiatric disorder and number of psychiatric diagnoses. The outcome variables, recorded both at trial entry and at follow-up, were number of days of restricted activity, SF-36 role limitation (physical) sub-scale score, and total costs. Age, gender, number of other medical conditions for which medication was being taken and severity of abdominal pain (recorded at baseline) were treated as possible confounders. All data were entered and analysed on the Statistical Package for the Social Sciences, version 11.5. The first, cross-sectional analysis compared the patients with and without each psychiatric diagnosis in terms of impairment measured at baseline (i.e. at the time the diagnosis was made) and with costs incurred over the previous year. These analyses used analysis of covariance (ANCOVA) with the possible confounders (listed above) as covariates.
Table 1 Number of patients and Hamilton Rating Scale for Depression scores for each psychiatric diagnosis

| Patients with diagnosis | Patients without diagnosis | Difference in HRSD scores | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | HRSD score | n | HRSD score | Mean | (95% CI) | |||
| Mean | (s.d.) | Mean | (s.d.) | |||||
| Depressive disorder | 74 (29) | 16.4 | (4.4) | 183 | 9.2 | (5.5) | 7.2 | (5.8 to 8.7) |
| Panic disorder | 30 (12) | 16.2 | (6.7) | 227 | 10.6 | (5.8) | 5.5 | (3.3 to 7.5) |
| Neurasthenia | 90 (35) | 13.9 | (6.1) | 167 | 9.9 | (5.7) | 4.0 | (2.5 to 5.5) |
| Hypochondriasis | 23 (9) | 12.5 | (5.6) | 234 | 11.2 | (6.2) | 1.4 | (−1.3 to 4.0) |
| Generalised anxiety disorder | 35 (14) | 14.8 | (4.8) | 222 | 10.7 | (6.2) | 4.1 | (1.9 to 6.2) |
| Any psychiatric disorder (ID 5+) | 121 (47) | 15.1 | (5.1) | 136 | 7.9 | (4.9) | 7.2 | (6.0 to 8.4) |
| Number of psychiatric diagnoses1 | ||||||||
| 0 | 78 | 7.5 | (5.1) | ANOVA | ||||
| 1 | 73 | 9.6 | (5.2) | |||||
| 2 | 57 | 14.5 | (5.3) | P <0.001 | ||||
| 3+ | 49 | 16.0 | (5.0) | |||||
Table 2 Demographic data and symptom patterns for patients with irritable bowel syndrome, with or without comorbid psychiatric disorder

| No psychiatric disorder (ID level <5) (n=136) | Psychiatric disorder (ID level 5+) (n=121) | Difference between (95% CI) | χ2 | P | |
|---|---|---|---|---|---|
| Demographic data, n (%) | |||||
| Gender | |||||
| Female | 108 (79) | 97 (80) | 0.023 | 0.88 | |
| Marital status | |||||
| Single | 27 (20) | 23 (19) | |||
| Married/cohabiting | 84 (62) | 85 (70) | 3.25 | 0.35 | |
| Separated/divorced | 21 (15) | 11 (9) | |||
| Widowed | 4 (3) | 2 (2) | |||
| Ethnicity | |||||
| White | 132 (97) | 121 (100) | 3.61 | 0.057 | |
| Education for > 12 years | 79 (58) | 61 (50) | 1.52 | 0.22 | |
| Middle class | 66 (50) | 51 (43) | 1.28 | 0.26 | |
| Unemployed through ill health | 27 (20) | 43 (35) | 7.95 | 0.005 | |
| Irritable bowel syndrome, n (%) | |||||
| Rome diagnosis | |||||
| General | 68 (50) | 56 (46) | 2.48 | 0.29 | |
| Diarrhoea | 42 (31) | 32 (26) | |||
| Constipation | 26 (19) | 33 (27) | |||
| Continuous variables, mean (s.d.) | |||||
| Age, years | 40.3 (12.6) (n=136) | 39.6 (11.0) (n=121) | 0.7 (−2.2 to 3.6) | 0.63 | |
| Duration of IBS, years | 11.0 (8.5) (n=136) | 10.2 (9.0) (n=121) | 0.8 (−1.4 to 2.9) | 0.49 | |
| Abdominal pain severity | 31.1 (24.6) (n=136) | 39.4 (25.4) (n=121) | 8.3 (2.1 to 14.4) | 0.009 | |
| Days with pain (out of past 30) | 23.7 (8.2) (n=134) | 24.8 (8.5) (n=120) | 1.1 (−3.1 to 1.0) | 0.30 | |
| Diary score | 1.62 (0.67) (n=122) | 1.75 (0.66) (n=104) | 0.13 (−0.05 to 0.30) | 0.16 | |
| Days restricted activity (past 12 months) | 121.4 (135.9) (n=136) | 172.9 (144.9) (n=121) | 51.5 (17.0 to 86.0) | 0.004 | |
| Number of other medical conditions | 0.54 (1.0) (n=136) | 0.93 (1.2) (n=121) | 0.4 (0.1 to 0.7) | 0.006 |
Multiple regression was used to identify the independent variables associated with number of days of restricted activity, SF-36 role limitation (physical) score and (log) costs. The variables entered into these regression analyses were: age, gender, marital status, years of education, number of other medical problems, abdominal pain severity (visual analogue scale), number of days with pain, and psychiatric disorder (depressive and panic disorders and neurasthenia). The expectation maximisation algorithm was used for missing data.
The prospective analyses assessed whether the psychiatric diagnosis recorded at trial entry was associated first, with the number of days of restricted activity and SF-36 role limitation (physical) score recorded at follow-up, and second, with costs incurred in the 12 months between the end of treatment and follow-up. These analyses used ANCOVA with treatment group (antidepressants, psychotherapy or treatment as usual) and baseline scores as covariates, in addition to the variables listed in the previous paragraph.
Patients with depressive or panic disorder were divided into two groups according to whether their HRSD score had dropped to 10 or below at 15 months' follow-up, indicating remission (Reference Frank, Prien and JarrettFrank et al, 1991). The resulting groups of ‘resolved’ and ‘unresolved’ depression were compared using ANCOVA with the same covariates as before. To assess whether there was a significant treatment by diagnosis effect, an analysis was performed which used the change in SF-36 role limitation score between trial entry and follow-up as the outcome variable. The ANCOVA included as covariates age, gender, number of other medical conditions, pain severity, baseline SF-36 role limitation score and treatment group. The first analysis assessed the effect of depressive disorder, treatment group (psychotherapy, antidepressants and treatment as usual) and the interaction of depressive disorder and treatment group. The second analysis included neurasthenia instead of depressive disorder.
RESULTS
Details of the trial have been published elsewhere (Reference Creed, Fernandes and GuthrieCreed et al, 2003) and only a summary is presented here. A total of 257 persons (81% of eligible patients) were recruited to the study. The 60 patients who declined to enter the study were similar in baseline characteristics to the participants. Of those who participated, 29% had the diarrhoea-predominant form of irritable bowel syndrome, 23% had the constipation-predominant form and 48% had the general form. During the 3 months prior to trial entry 93% were using antispasmodic medications, 26% used antidiarrhoeal medication and 38% were taking one or more additional analgesics; 60% were taking laxatives and 16% motility stimulants; 29% had used alternative therapies previously. The median duration of bowel problems was 8 years and many patients had been attending the gastroenterology clinics for years prior to entry into this study. Abdominal pain occurred on most days (mean 24 days per month) and led to restricted activity on average 12 days per month. Thirty-four per cent were taking medication for one medical condition and 24% for two or more conditions. Fifty-nine per cent were in full-time or part-time employment, 5% were unemployed but seeking work and 27% were unemployed through illness; the remainder were retired, homemakers or students.
Psychiatric disorders
Almost half of the participants (121; 47%) were classified by the SCAN programme as reaching the threshold for psychiatric disorder (ID level 5 or above). The numbers of patients assigned to each diagnosis is shown in Table 1. The diagnoses not shown were dysthymia 17 (7%), phobias 39 (15%), undifferentiated somatoform disorder 24 (9%) and drug or alcohol problems 21 (8%). Many patients had more than one disorder: for example, of the 74 patients with depressive disorder, 16 also had panic disorder, 6 had hypochondriasis and 41 had neurasthenia.
Associated features of psychiatric disorders
Patients with psychiatric disorder (ID level 5 or above) did not differ significantly from the remainder with socio-demographic features or symptom type, but they had more other medical conditions and more severe abdominal pain (Table 2). Those with neurasthenia were younger, and SF-36 role limitation scores were higher in women. All subsequent analyses were therefore adjusted for age, gender, number of other medical conditions and severity of abdominal pain.
Psychiatric disorders and outcome measures at baseline
At baseline, depressive disorders, panic disorders and neurasthenia were associated with both restricted activity and impaired role functioning; depressive and panic disorders were associated with increased total costs (Table 3). No other psychiatric diagnosis was associated significantly with these outcomes. Of the 70 people unable to work owing to illness, 30 (43%) had depressive disorder, compared with 44 out of 187 (24%) of those able to work (OR=2.4, 95% CI 1.4-4.4), and 17 (24%) had panic disorder, compared with 13 out of 187 (7%) of those able to work (OR=4.3, 95% CI 2.0-9.4). No other psychiatric disorder was associated with unemployment through ill health.
Table 3 Number of days with restricted activity, SF-36 role limitation (physical) score and total costs for year prior to entry to the trial, by diagnosis

| n | Days of restricted activity (past year) | SF-36 role limitation (physical score) | Total costs including productivity, £ | ||||
|---|---|---|---|---|---|---|---|
| Baseline n=257 | Baseline n=250 | Baseline n=249 | |||||
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | ||
| Depressive disorder | |||||||
| Yes | 74 | 178.7 | (147.8-209.6) | 24.4 | (15.8-32.9) | 1802 | (1410-2193) |
| No | 183 | 132.3 | (112.8-151.7) | 41.9 | (36.5-47.4) | 1111 | (867-1354) |
| P=0.0141 | P=0.0011 | P=0.0041 | |||||
| Panic disorder | |||||||
| Yes | 30 | 213.3 | (165.1-261.5) | 15.5 | (1.8-29.1) | 2216 | (1609-2822) |
| No | 227 | 136.7 | (119.4-184.0) | 39.5 | (34.6-44.4) | 1186 | (969-1403) |
| P=0.0041 | P=0.0011 | P=0.0021 | |||||
| Neurasthenia | |||||||
| Yes | 90 | 181.6 | (153.8-209.3) | 24.9 | (17.1-32.6) | 1311 | (953-1670) |
| No | 167 | 126.3 | (106.0-146.5) | 43.2 | (37.5-48.9) | 1303 | (1044-1562) |
| P=0.0021 | P < 0.00051 | P=0.971 | |||||
At baseline the number of psychiatric disorders was associated with number of days of restricted activity, SF-36 role limitation (physical) score and total costs in a dose-response fashion (Figs 1, 2, 3).
Predictors of impairment and costs at baseline
Table 4 displays a summary of the regression analyses to determine the variables most closely associated with impairment and costs at baseline. Severity of abdominal pain was the most strongly associated predictor of all outcomes. Panic disorder and neurasthenia were also independent predictors of both measures of impairment (days of restricted activity and role limitation score); depressive disorder was associated with role limitation score and costs.
Table 4 Results of multiple regression analyses

| Dependent variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days of restricted activity (n=257) | SF-36 score (n=250) | Log(total costs including productivity) | |||||||
| Independent variables | B | (s.e.)1 | P 2 | B | (s.e.)1 | P 2 | B | (s.e.)1 | P 2 |
| Age | − 0.7 | (0.87) | 0.42 | − 0.7 | (0.2) | 0.007 | − 0.004 | (0.003) | 0.11 |
| Female gender | − 70.5 | (20.9) | 0.001 | 9.2 | (5.9) | 0.12 | − 0.010 | (0.066) | 0.88 |
| Single | 2.7 | (23.4) | 0.91 | − 5.2 | (6.5) | 0.42 | − 0.070 | (0.074) | 0.35 |
| Widowed, separated or divorced | 11.7 | (23.5) | 0.62 | 0.7 | (6.6) | 0.92 | 0.055 | (0.074) | 0.46 |
| Educated > 12 years | − 50.3 | (18.1) | 0.006 | − 6.8 | (5.1) | 0.18 | − 0.047 | (0.057) | 0.41 |
| Number of other medical conditions | 11.5 | (7.4) | 0.12 | − 1.4 | (2.1) | 0.49 | 0.053 | (0.023) | 0.022 |
| Abdominal pain severity | 0.9 | (0.4) | 0.011 | − 0.2 | (0.1) | 0.018 | 0.004 | (0.001) | 0.002 |
| Days with pain (out of past 30) | 0.4 | (1.1) | 0.74 | − 0.3 | (0.3) | 0.27 | 0.003 | (0.003) | 0.44 |
| Years of IBS | 1.1 | (1.0) | 0.27 | 0.1 | (0.3) | 0.62 | 0.000 | (0.003) | 0.91 |
| Depression | 28.9 | (19.2) | 0.13 | − 11.3 | (5.4) | 0.035 | 0.149 | (0.061) | 0.015 |
| Neurasthenia | 55.8 | (26.4) | 0.035 | − 17.5 | (7.5) | 0.020 | 0.101 | (0.084) | 0.23 |
| Panic disorder | 46.0 | (18.0) | 0.011 | − 13.1 | (5.1) | 0.010 | − 0.039 | (0.057) | 0.49 |
| Constant | 163.6 | (52.4) | 0.002 | 87.0 | (14.8) | <0.001 | 3.070 | (0.166) | <0.001 |
| Adjusted R 2 (%) | 17.4 | 16.4 | 10.6 | ||||||
Does psychiatric disorder at baseline predict outcome 15 months later?
Of the three psychiatric diagnoses associated with impairment at baseline, only one - neurasthenia - was significantly associated with number of days per month of restricted activity at follow-up: 11.7 days (s.e.m.=1.1) compared with 7.5 (s.e.m.=0.8) for the patients without neurasthenia (P=0.004, adjusted for treatment group as well as age, gender, number of medical conditions, severity of abdominal pain at baseline and baseline value of days of restricted activity). Neurasthenia and depressive disorder were both associated with SF-36 role limitation (physical) score at follow-up: neurasthenia: 37.1 (s.e.m.=4.5) v. 53.5 (s.e.m.=3.2), adjusted P=0.004; depressive disorder: 35.3 (s.e.m.=4.9) v. 53.1 (s.e.m.=3.1), adjusted P=0.003; (P adjusted for similar covariates, except baseline value of SF-36 role limitation was used instead of days of restricted activity). The number of psychiatric disorders at baseline predicted SF-36 role limitation score at follow-up (Fig. 2) but not days of restricted activity or costs (Figs 1, 3).

Fig. 1 Number of days of restricted activity during previous month at baseline and at follow-up by number of psychiatric diagnoses.
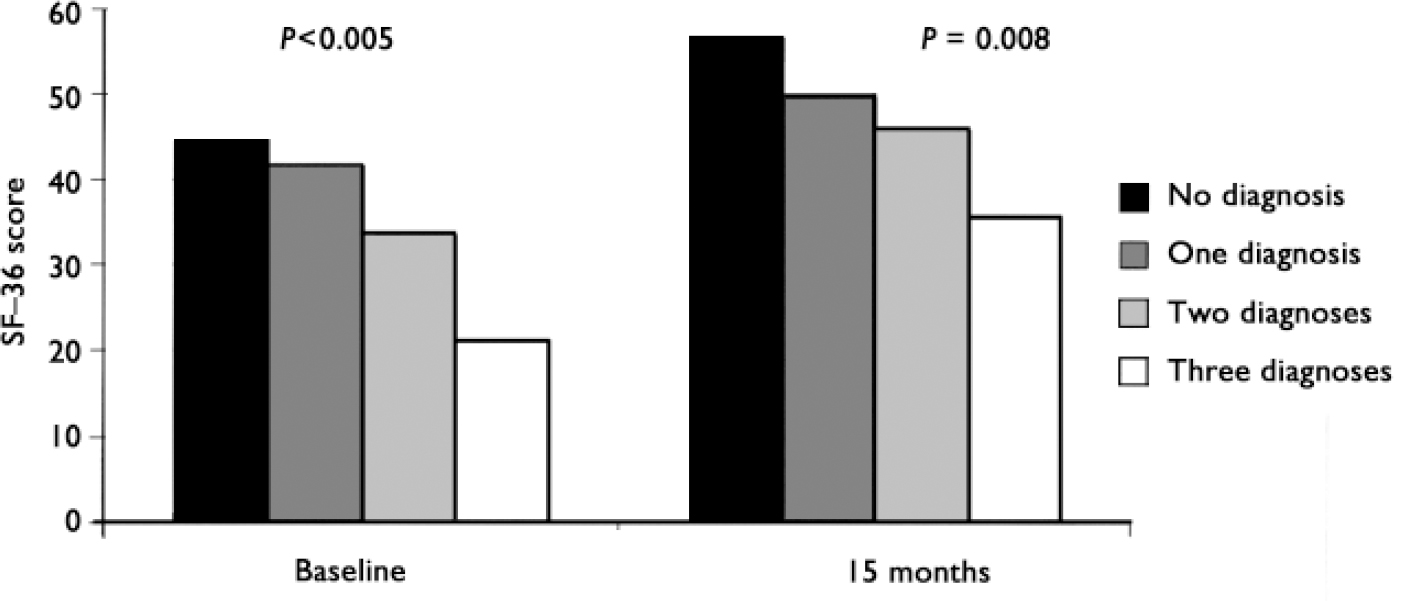
Fig. 2 Scores on the 36-item Short Form Health Survey (SF-36) physical role limitation sub-scale at baseline and at follow-up by number of psychiatric diagnoses.

Fig. 3 Healthcare costs for year before baseline and for year prior to follow-up by number of psychiatric diagnoses.
Change over 15 months of the trial
Of the 82 patients with depressive and/or panic disorder at baseline, 35 had an HRSD score of 10 or less at follow-up. This resolved group had an adjusted HRSD score at baseline of 14.9 (95% CI 13.1-16.6), compared with a baseline adjusted HRSD score of 17.0 (95% CI 15.5-18.5) for the unresolved group (n=47); P=0.052. At follow-up the HRSD score for the resolved group had fallen by approximately 10 points to an adjusted mean of 5.0, whereas the adjusted mean score of the unresolved group remained the same (Table 5). The resolved group experienced significantly fewer days of restricted activity than the unresolved group during the month prior to follow-up assessment. The SF-36 role limitation score showed significantly less impairment in the resolved group than in the unresolved group but there was no difference in overall costs (Table 5).
Table 5 Analysis of covariance for patients with depressive or panic disorder at baseline divided according to resolution of depression at follow-up

| Outcome variable | Resolved group (HRSD score ≤10) | Unresolved group (HRSD score ≥11) | Adjusted P value (ANCOVA)1 | ||||
|---|---|---|---|---|---|---|---|
| Mean (95% CI) | s.e.m. | n | Mean (95% CI) | s.e.m. | n | ||
| HRSD score at follow-up | 5.0 (3.8-6.3) | 0.6 | 35 | 17.1 (16.0-18.2) | 0.5 | 47 | |
| Days of restricted activity during month before follow-up | 8.5 (4.6-12.4) | 1.9 | 35 | 14.0 (10.7-17.4) | 1.7 | 46 | 0.043 |
| SF-36 role limitation (physical) score at 15 months | 49.7 (36.9-62.5) | 6.4 | 32 | 16.3 (5.3-27.3) | 5.5 | 42 | <0.001 |
| Total costs including productivity (3-15 months) £/week | 30.5 (19.2-41.7) | 5.6 | 35 | 31.1 (21.3-41.0) | 5.0 | 44 | 0.93 |
Changes in SF-36 score with treatment
The results of the two-way ANCOVA assessing the effect of depressive disorder and treatment group indicated that there was a significant effect of depressive disorder (Fig. 4). For the 64 patients with depression the adjusted mean change in the SF-36 physical role limitation sub-scale score was -1.6 (s.e.m.=4.9); the corresponding score for the 155 patients without depression was 17.2 (s.e.m.=3.1); P=0.002. The difference between treatment groups was of borderline significance: psychotherapy 10.4 (s.e.m.=5.1), paroxetine 14.8 (s.e.m.=4.6) and treatment as usual -1.79 (s.e.m.=5.0); P=0.049. There was no significant treatment × depression interaction (P=0.28).
The comparable results for neurasthenia showed a significant effect of diagnosis: mean change score 0.98 (s.e.m.=4.6) for neurasthenia v. 17.1 (s.e.m.=3.2) for the remainder; P=0.005 (Fig. 5). The treatment effect was also significant: 15.6 (s.e.m.=4.7) for psychotherapy, 13.0 (s.e.m.=4.4) for paroxetine and -1.5 (s.e.m.=5.1) for treatment as usual respectively; P=0.034. There was no significant treatment × neurasthenia interaction effect (P=0.52).
DISCUSSION
This is the first study to show the adverse effect that individual psychiatric disorders have on outcome in people with severe irritable bowel syndrome. Panic and depressive disorders and neurasthenia were associated with greater impairment at trial entry; the first two disorders were also associated with increased costs during the previous year. At follow-up 15 months later, depressive disorder and neurasthenia were associated with impaired role function. Both depressive disorder and neurasthenia were associated with lack of improvement in SF-36 role limitation sub-scale scores between trial entry and follow-up. No psychiatric disorder was associated with total costs during the follow-up year. The dose-response relationship between number of psychiatric disorders and days of restricted activity and SF-36 role limitation score suggests a causal relationship. This is also supported by our finding that reduction in depressive symptoms at follow-up was associated with reduction in days of restricted activity and improved SF-36 role limitation scores. Our multiple regression analyses demonstrated that depressive and panic disorders and neurasthenia were independently associated with baseline measures of impairment even after the severity of abdominal pain and other irritable bowel symptoms (recorded in the daily diary) had been accounted for. These findings support our principal hypotheses concerning the association between psychiatric disorders and impairment of daily function, but offer less support for a direct association with costs.
The psychiatric disorders we identified in our participants were mild in intensity compared with those usually treated by psychiatrists or included in trials of depressive disorder. However, the adverse effect on outcome is important. Impairment of health-related quality of life in irritable bowel syndrome has been reported by others as being comparable with that occurring in gastro-oesophageal reflux disease, diabetes and heart disease (Reference Naliboff, Balice and MayerNaliboff et al, 1998; Reference Drossman, Whitehead and TonerDrossman et al, 2000; Reference Gralnek, Hays and KilbourneGralnek et al, 2000; Reference LuscombeLuscombe, 2000; Reference Lea and WhorwellLea & Whorwell, 2001; Reference El-Serag, Olden and BjorkmanEl-Serag et al, 2002), but only two of these six reports have acknowledged that this impairment could be attributed, in part, to co-existing psychiatric disorder (Reference Naliboff, Balice and MayerNaliboff et al, 1998; Reference Drossman, Whitehead and TonerDrossman et al, 2000).

Fig. 4 Changes in scores on the 36-item Short Form Health Survey (SF-36) role limitation sub-scale by treatment group and presence of depressive disorder (negative score represents deterioration).

Fig. 5 Changes in scores on the 36-item Short Form Health Survey (SF-36) role limitation sub-scale by treatment group and presence of neurasthenia (negative score represents deterioration).
Our study draws attention to the component of impairment that is potentially treatable by psychological means. We found that patients with depressive or neurasthenic disorders who did not receive psychotherapy or paroxetine tended to show a worsening of their SF-36 role limitation scores. If psychiatric disorders are not recognised and treated in this population, people with severe irritable bowel syndrome may suffer unduly and miss time from their work or usual household activities. Since the syndrome was so long-standing it is likely that many patients had had untreated psychiatric disorders for months or even years.
Our sample was particularly impaired because of the way the participants were recruited. Even those without a coexisting psychiatric disorder had low SF-36 scores, reflecting severe abdominal pain and bowel disturbance (Reference Hahn, Kirchdoerfer and FullertonHahn et al, 1997). For patients with psychiatric disorder the very low SF-36 scores were similar to those previously reported in studies of severe comorbid physical and psychiatric disorders (Reference Ware, Kosinski and KellerWare et al, 1994; Reference Creed, Morgan and FiddlerCreed et al, 2002). Like others, we have found that this combination of physical and psychiatric disorder leads to high costs because of time missed from work and expensive healthcare (Reference Druss, Rosenheck and SledgeDruss et al, 2000).
The strengths of the study are its large sample size, its representative nature (81% of eligible patients were recruited), the detailed measures and its prospective design. We were able to measure healthcare costs in an objective manner using NHS records, but we have quoted only total costs (i.e. healthcare and loss of productivity) for the sake of brevity. Our measures of impairment of daily living were self-reported, but great care was taken to record days of restricted activity accurately as part of the costing exercise. We have reported elsewhere that SF-36 scores are significantly associated with time off work, which provides some evidence of the validity of this self-report measure (Reference Creed, Fernandes and GuthrieCreed et al, 2003). The prospective design was limited by the fact that two-thirds of patients received either antidepressants or psychotherapy, but we were able to show no treatment by diagnosis interaction.
The adjustment for severity of abdominal pain was important because this was the principal predictor of impaired function and costs at baseline (see Table 4). It allowed us to identify the component of impairment that could be attributed to coexisting psychiatric disorders. We might have reduced the strength of the association, however, because there was a significant association between psychiatric disorder and abdominal pain severity score. Our results may provide a conservative estimate of the association between psychiatric disorder and outcome. A further theoretical consideration lies in the possibility that the main predictor of outcome is the total number of symptoms (physical and psychological) and that irritable bowel syndrome and depressive, panic and neurasthenic disorders might be seen as overlapping disorders. Although this is possible, the fact that severity of abdominal pain and psychiatric disorders contribute independently towards health-related quality of life argues for independent influences of these syndromes.
We chose to use the single SF-36 scale of physical limitation because of its lack of close correlation with neuroticism and its close correlation with patients' views of the disabling nature of severe irritable bowel syndrome. We found that it was the sub-scale (of eight) that most closely correlated with days of restricted activity at baseline and at follow-up (data not shown). The only scale that correlated more highly was the physical component summary score, but we could not use this score because it is a composite scale which includes a measure of pain, so that adjustment for severity of pain would have been unsatisfactory.
There were three main weaknesses of this study. First, this is a secondary analysis of a data-set that was collected for another purpose and patient numbers are not large enough to test adequately all the questions regarding healthcare and productivity costs. Second, although our sample was representative of patients with severe irritable bowel syndrome in gastroenterology clinics, the results cannot be generalised to all patients with this syndrome, most of whom have milder forms of the disorder. Third, because our patients were in a trial, two-thirds received an intervention, which has affected outcome; this is not a naturalistic study. Our study might have been underpowered to detect a true treatment by diagnosis interaction.
The diagnosis of neurasthenia is similar to that of chronic fatigue syndrome, and one other paper has reported that the presence of chronic fatigue in patients with irritable bowel syndrome is associated with impaired health-related quality of life and poor outcome (Reference Simren, Ambrahamsson and SvedlundSimren et al, 2001). It is recognised that chronic fatigue syndrome, fibromyalgia and psychiatric disorders often occur together with irritable bowel syndrome (Reference Whitehead, Palsson and JonesWhitehead et al, 2002); such comorbidity is associated with greater impairment than when the syndromes occur singly (Reference Sperber, Carmel and AtzmonSperber et al, 2000). Neurasthenia is one of the most disabling conditions in population studies and the number of days of restricted activities increased with increasing number of comorbid conditions (Reference Andrews, Sanderson and BeardAndrews et al, 1998).
The mechanisms by which depressive, panic and neurasthenic disorders lead to increased impairment and healthcare costs are not totally clear. Because we controlled for the effect of pain and other medical conditions, these cannot be explanations for high healthcare and other costs as suggested previously (Reference Simon, Von Korff and BarlowSimon et al, 1995). It is likely that psychiatric disorders act in several ways: they may lead to worsening of pain and bowel dysfunction, they may exacerbate worry about illness and bodily symptoms (Reference Gomborone, Dewsnap and LibbyGomborone et al, 1995) and they may reduce motivation to perform daily tasks.
The major implication of these results is that clinicians should consider whether psychiatric disorders are present in patients with severe irritable bowel syndrome, and if so, appropriate treatment should be offered. Recommendations for treatment of the severe syndrome suggest psychological or behavioural treatments, rather than medication directed towards relieving gut symptoms (American Gastroenterology Association, 2002). This has major implications for healthcare provision. Gastroenterologists or primary care physicians should be prepared to screen all such patients for psychiatric disorders and suitably trained health professionals need to be available for complex cases. The current referral pattern of highly selected patients within the traditional consultation-liaison model is unlikely to address adequately the unmet needs of these patients (Reference Lloyd and MayouLloyd & Mayou, 2003). The professional background of the mental health professional is less important than readiness to see these patients, who appear to respond equally well to antidepressants prescribed by a gastroenterologist or general practitioner, or psychotherapy administered by a suitably trained therapist (Reference Creed, Fernandes and GuthrieCreed et al, 2003).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ An important component of impairment of health-related quality of life in patients with severe irritable bowel syndrome can be attributed to comorbid psychiatric disorder.
-
▪ Psychotherapy or antidepressant treatment with a selective serotonin reuptake inhibitor leads to improved functioning in this population.
-
▪ The current system of liaison psychiatry is unlikely to meet this need adequately; close collaboration between psychologists or psychiatrists and gastroenterologists is required.
LIMITATIONS
-
▪ The study is a secondary analysis of data collected as part of a randomised controlled trial.
-
▪ The results cannot be generalised to all patients with possibly milder irritable bowel syndrome.
-
▪ The study might have been underpowered to detect treatment by diagnosis interaction.
Acknowledgements
The research team thank the UK Medical Research Council for financing the study, the North Western and Yorkshire Regional Health Authorities for financing the psychotherapists, the patients who consented to take part in the trial and the doctors who prescribed the antidepressant medication. The study was supported by grants from the Medical Research Council and the North Western Regional Health Authority Research & Development Directorate. SKB provided the paroxetine but were not involved in the design, conduct or analysis of the study. The North of England IBS Research Group consists of Chris Babbs, Joe Barlow, Karna Dev Bardham, Francis Creed, David Dawson, Lakshmi Fernandes, Elspeth Guthrie, Stephanie Howlett, Linda McGowan, Jane Martin, Jim Moorey, Kieran Moriarty, Stephen Palmer, Joy Ratcliffe, Nicholas Read, Wynne Rees, Christine Rigby, Irene Sadowski, Jon Shaffer, David Thompson, Barbara Tomenson and Pierre Willemse.













eLetters
No eLetters have been published for this article.