Neuropsychiatric symptoms (NPS) represent a common and important manifestation of the dementia syndrome, with major effects on quality of life, carer burden and risk of institutionalisation.Reference van der Linde, Dening, Stephan, Prina, Evans and Brayne1 Although non-pharmacological interventions are a good first-line option, there are few treatment options for the more severe and persistent symptoms, and clinical trials are hampered by a large placebo effect, which could be due to natural fluctuations of symptoms.Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris and Patel2 The longitudinal course of NPS is only partly known. Longitudinal studies have often been based on small sample sizes of mixed dementia groups, and with a short duration of follow-up. Studies usually report summary data, i.e. the proportion of patients with NPS at each time point.Reference van der Linde, Dening, Stephan, Prina, Evans and Brayne1,Reference Selbaek, Engedal and Bergh3,Reference Haaksma, Leoutsakos, Bremer, Aalten, Ramakers and Verhey4 However, the different symptoms may vary in each patient, from single occurrence over a short period, via a fluctuating course with remission and relapse, to a persistent course, and thus the proportion with a symptom at each time point does not inform about the course of NPS in individual patients. For example, in our recent 5-year study, we showed that although most NPS were common already from the time of diagnosis, there were individual fluctuations, and thus the group with the symptom present consisted of different individual patients at different time points.Reference Vik-Mo, Giil, Ballard and Aarsland5 In one of the longest studies to date, following patients for up to 9 years, aggression was found to be persistent, whereas other NPS occurred as single discrete episode.Reference Hope, Keene, Fairburn, Jacoby and McShane6 Understanding the course of NPS in individual patients is important for precision medicine treatment approach and planning, as well as for trial design. Here, we present the individual course for up to 12 years from diagnosis of dementia to death in people with Alzheimer's disease and Lewy body dementia (LBD).
Method
Study design
The Dementia Study of Western Norway (Demvest) is a longitudinal cohort study of patients referred to dementia clinics in Hordaland and Rogaland counties. There is little private healthcare for these patients and all dementia units (i.e. geriatric, neurology and geriatric psychiatry out-patient clinics) in the region were recruited to the study. To reduce referral bias, the general practitioners in the area were contacted by letter before study start and were invited to refer all patients with suspect dementia. Residents are covered by the same National Insurance Scheme with restricted co-payments, allowing the representation of a general dementia population. All patients referred with suspected mild dementia were screened (n = 657), 325 fulfilled the inclusion criteria and 223 consented to the study. After the main inclusion period between 2005 and 2007, we continued to selectively recruit patients with LBD (i.e. dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD)) to enhance the number of patients in this group.
Procedure
Criterion for inclusion was mild dementia according to the ICD-10,7 defined as a Mini-Mental State Examination (MMSE)Reference Folstein, Folstein and McHugh8 score of at least 20 or a Clinical Dementia Rating (CDR) global score of 1, were included. Exclusion criteria were moderate or severe dementia, acute delirium, previous bipolar disorder or psychotic disorder, terminal illness or recently diagnosed major somatic illness that, according to the clinician, would significantly affect cognition, function or study participation. The standardised diagnostic assessment is described in detail elsewhere.Reference Aarsland, Rongve, Nore, Skogseth, Skulstad and Ehrt9 Briefly, physical, neurological and psychiatric examinations were performed, including a detailed neuropsychological test battery, Montgomery–Aasberg Depression Rating scale,Reference Montgomery and Asberg10 routine blood and cerebrospinal fluid analyses and brain magnetic resonance imaging. Dopamine transporter single-photon emission computed tomography scans were available for most patients with suspected DLB. Caregivers completed The Informant Questionnaire on Cognitive Decline in the Elderly, a questionnaire shown to be a reliable and valid instrument to detect dementia, and the clinician completed the CDR and the Hachinski Ischemia Scale.Reference Hachinski, Iliff, Zilhka, Du Boulay, McAllister and Marshall11–Reference Morris13
The clinical diagnoses were reviewed by a consensus group at regular intervals, taking into account all available information, including the electronic medical records. The final clinical diagnosis was made according to the consensus criteria for dementia with LBD, PDD and Alzheimer's disease after a consensus meeting with three specialists, including both geriatric psychiatry and geriatric medicine. A pathological diagnosis was available for 56 patients, showing diagnostic accuracy above 80% for both Alzheimer's disease and LBD.Reference Skogseth, Hortobagyi, Soennesyn, Chwiszczuk, Ffytche and Rongve14 Patients were followed with annual structured assessments. The participating centres follow national guidelines on psychotropic use, offer dementia-carer support groups, practice patient-centred care and provide ambulatory services to nursing homes.
Assessment and classification of NPS
The validated Norwegian Neuropsychiatric Inventory (NPI) was used to interview to family or caregivers, and the nursing home version NPI-Nursing Home was used after participants moved to nursing homes.Reference Selbaek, Kirkevold, Sommer and Engedal15,Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein16 All assessments were completed by the best-suited informants who had the most day-to-day contact with the patient. Informants were the spouse, children or, at later assessments, a professional caregiver. The 12 items were registered as present or not present during the past 4 weeks, and if present, scored according to their frequency (1–4) and severity (1–3). Here, we report the frequency × severity score for the individual items. We present data in Table 3, using the established cut-off item score of ≥4 to indicate a clinically significant symptom, which includes moderately severe symptoms present at a frequency rating of ‘often’ or more frequently, and mild symptoms present ‘very frequently,’ as previously reported.Reference van der Linde, Dening, Stephan, Prina, Evans and Brayne1,Reference Brodaty, Connors, Xu, Woodward and Ames17 In addition, results for NPI item scores present (≥1), severe (≥8) and very severe (12) are presented in Supplementary Table 1 available at https://doi.org/10.1192/bjp.2019.195. For NPI total score, the cut-offs were set and classified as NPS ≥1 (present), ≥12 (modest severity), ≥24 (significant) and ≥48 (very significant) (Table 2).
The clinical course was coded into four mutually exclusive categories defined as follows: no symptoms, never having symptom above the relevant cut-off; stable, symptom present at all or three consecutive assessments; relapsing, symptom present at two or more assessments but with resolution of symptom between assessments; single episode, symptom only present at one assessment during follow-up.
Statistics
Clinical and demographic variables are shown as mean or proportions and statistical differences are tested with Mann–Whitney and Student's t-test (in Table 1). Differences between Alzheimer's disease and LBD frequency of symptoms (no/yes, Table 3) and clinical course were tested with the χ2 test, as contingency tables. All analysis was done in SPSS (version 13 for Windows).
Table 1 Clinical and demographic variables

LBD, Lewy body dementia; t/z, Standard score test statistics; CIRS, Cumulative Illness Rating Scale; CDR-SB, Clinical Dementia Rating sum of boxes; MMSE, Mini-Mental State Examination.
a. Mann–Whitney/ Student's t-test showing t-score for Alzheimer's disease and LBD.
b. Pearson's χ2 test, showing z-score for Alzheimer's disease and LBD.
Ethics
The study was approved by the regional ethics committee (approval no. 2010/633). All participants signed informed consent at study start when they had mild dementia and capacity to consent. Next-of-kin provided signed informed consent as well. We received financial support only from the regional health authorities of western Norway, Helse-Vest and non-profit organisation Norwegian Health Association. All data were handled and kept in accordance with national health and data privacy protocol.
Results
Clinical and demographic variables
The cohort consisted of 113 patients with Alzheimer's disease and 84 patients with LBD (including 16 patients with PDD). Ten patients were still alive in January 2018, all of whom had completed the 12-year follow-up. The mean duration of follow-up was 6.4 (s.d. 2.9) and 4.3 (s.d. 1.9) years for Alzheimer's disease and LBD, respectively. There were 1080 possible assessments (living patient-years), with a mean number of observations of 4.96 (s.d. 2.3) and total of 1063 completed observations. The attrition and missing rates for reasons other than death were very low; only 19 patients missed one single follow-up assessment and two missed two assessments, leading to 98% total completeness of the longitudinal data. The characteristics of patients with Alzheimer's disease and LBD did not differ regarding age, education or baseline MMSE score (Table 1), but the Alzheimer's disease group included more women and had a shorter duration of symptoms compared with the LBD group. We have previously reported a shorter survival time and more rapid disease progression in DLB.Reference Rongve, Soennesyn, Skogseth, Oesterhus, Hortobagyi and Ballard18,Reference Oesterhus, Soennesyn, Rongve, Ballard, Aarsland and Vossius19 The drug use in the cohort at inclusion was reported previously, and 14% had ‘potentially inappropriate’ and potentially severe drug–drug interactions.Reference Oesterhus, Aarsland, Soennesyn, Rongve, Selbaek and Kjosavik20 These relatively low proportions indicate that the prescribing practice was acceptable.Reference Oesterhus, Aarsland, Soennesyn, Rongve, Selbaek and Kjosavik20 At the first follow-up, 61% used antidementia drugs, 9% used antipsychotics and 40% used antidepressants.
NPI total course
Only one patient was without NPS, but the longitudinal course of the NPI total score varied considerably among patients. The proportion of patients having no, stable, relapsing or single episode courses of the different NPI total score categories are shown in Table 2. Most patients (80%) had stable NPS total (≥1), whereas 50% had stable modest (NPI total ≥12) and 25% stable significant (NPI total ≥24) NPI total scores. Only five patients (four with Alzheimer's disease) had year-to-next-year increase in the first three follow-up assessments, and only eleven (all with Alzheimer's disease) had four of five year-to-next-year increases. All of these patients had modest NPI total score (<24), and most scores were <12. A minor group of both patients with Alzheimer's disease and LBD (5%) experienced few episodes, and with only mild NPS (Fig. 1). Very severe symptoms (NPI total ≥48) were mostly single episodes, but 8% of patients with Alzheimer's disease had stable, very severe symptoms. Patients with LBD had mild and moderate NPS more often, but more rarely had severe and stable NPS (1%).
Table 2 The clinical course of Neuropsychiatric Inventory (NPI) total score in Alzheimer's disease and Lewy body dementia at different severity levels

The clinical course was coded into four different categories defined at different cut-offs (NPI total ≥1, ≥12, ≥24 and ≥48): no symptom, never having symptom; stable, symptom being present at all or three consecutive assessments; single episode, symptom only present at one assessment during follow-up; relapsing, symptoms present at two or more assessments but with resolution of symptom between assessments.
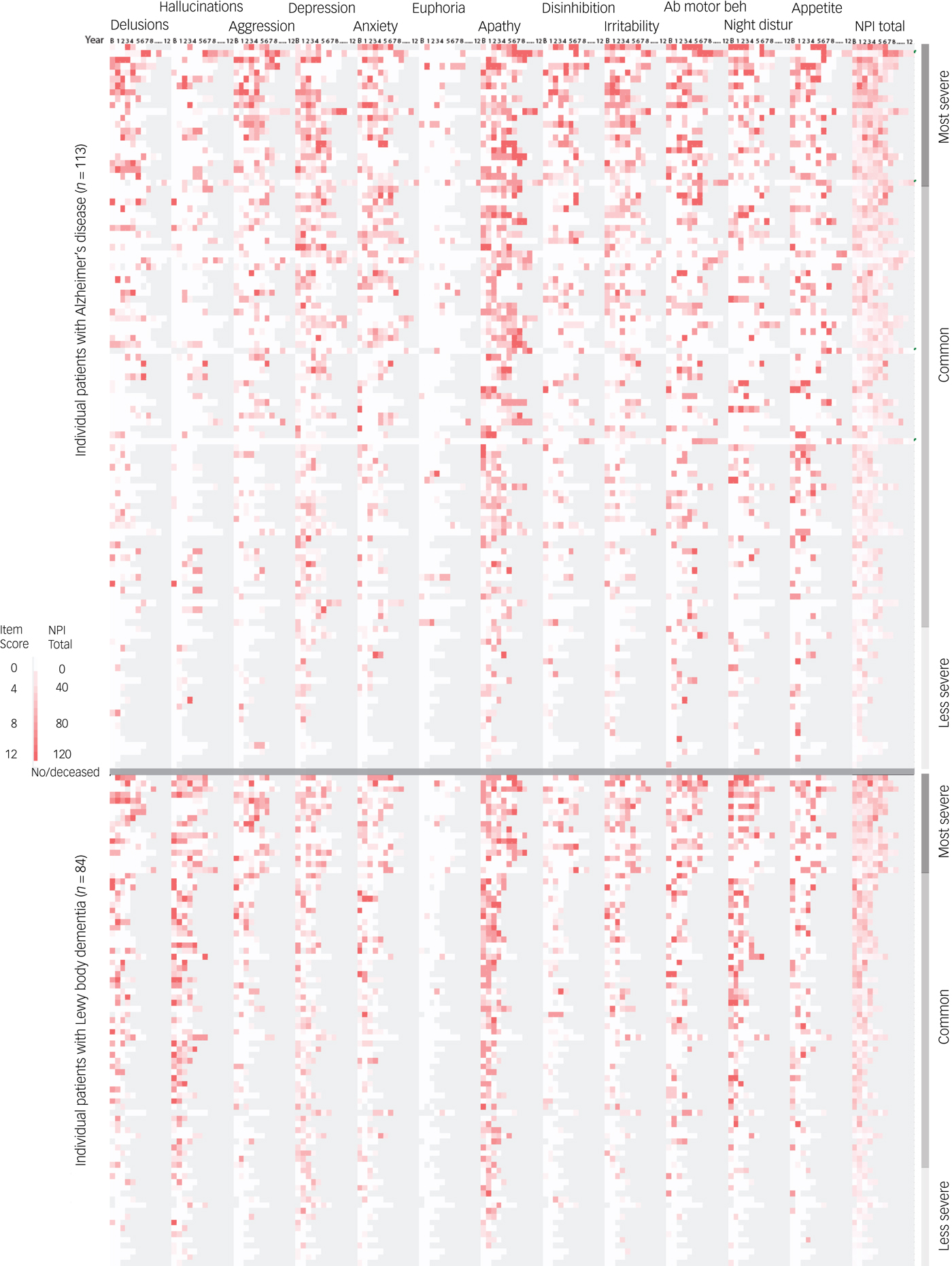
Fig. 1 Heatmap of Neuropsychiatric Inventory (NPI) scores at all assessments for Alzheimer's disease and Lewy body dementia sorted by NPI total score. All assessments are shown as a heatmap graded from 0 to 12 based on items score. The NPI total score is graded on the full scale, 0–144. Patient death and all missing data is shown in grey. The patients are sorted by diagnosis and highest cumulative NPI total score. To exemplify, the patient with Alzheimer's disease with the highest NPI total score (first case) died after seven follow-up assessments. He had mild delusions at baseline and more severe at years four and five (relapsing course), hallucination only at year five (single episode) and aggression from year three to seven (stable course). He also had a stable course of significant neuropsychiatric symptoms (NPI total score ≥24), but only relapsing very significant neuropsychiatric symptoms (NPI total score ≥48).
Course of individual NPS
The majority of patients had many single episodes or relapsing individual NPS. This is illustrated by the irregular spotted pattern shown on the heatmap (Fig. 1), best depicted by depression and anxiety. Some patients had complete symptom resolution late in the course, even in patients with severe symptoms. Relapsing or single episode patterns were more pronounced among the higher item scores (Supplementary Table 1). Apathy was the most stable symptom in both Alzheimer's disease and LBD groups, with 34 and 27% of patients having stable apathy, respectively. Anxiety and depression had relatively low persistency in both patients with Alzheimer's disease and LBD, i.e. 35% of patients with LBD (60% of symptomatic patients) had only a single episode of depression symptoms. Anxiety, irritability and aberrant motor behaviour were more common and relatively more stable in the Alzheimer's disease group, with 14, 18 and 27% having a stable course, respectively ((Table 3; χ2 P < 0.05 compared with LBD).
Table 3 Clinical course of neuropsychiatric symptoms in Alzheimer's disease and Lewy body dementia

Symptoms assessed annually with the Neuropsychiatric Inventory (NPI). The clinical course was coded into four different categories defined in order: no symptom, never having symptom above set cut-off of ≥4; stable, symptom being present at all or three consecutive assessments; single episode, symptom only present at one assessment during follow-up; intermediary, symptoms present at two or more assessments but with resolution of symptom between.
Statistical test Alzheimer's disease versus Lewy body dementia: *P < 0.05 χ2 test no/yes symptoms present, **P < 0.001 χ2 test no/yes symptoms present.
With increasing NPI item scores, the number of patients having stable and relapsing courses significantly decreased, with an increase in single episodes (Supplementary Table 1). In Alzheimer's disease, the mean risk of having single episode increased from 31% (s.d. 12%) with NPI score ≥1 to 62% (s.d. 14%) with NPI score ≥8 (paired Student's t-test, P ≥ 0.001). This effect was similar in LBD. In Alzheimer's disease, of the 496 NPI scores ≥8, 54% (270 of 496) were single episodes, similar to LBD at 43% (193 of 318, P = 0.081). There was a significant difference between groups in the course of patients scoring a maximum NPI item score of 12, with 72% (154 of 213) of patients with Alzheimer's disease having these as single episodes, compared to 83% (98 of 117) of patients with LBD (χ2 P = 0.032).
Psychotic symptoms
Seventy-nine patients with LBD (94%) and 87 patients with Alzheimer's disease (77%) experienced at least one psychotic symptom (NPI ≥1 of delusions or hallucinations). In LBD, 83% had reoccurring psychotic symptoms, compared with 57% with psychosis in Alzheimer's disease. Clinically significant hallucinations (NPI ≥4) had a stable course in 24% in LBD but only 4% in Alzheimer's disease. Hallucinations usually occurred rather early in the course, but some patients with Alzheimer's disease developed late hallucinations (Fig. 1). The percentage of patients with LBD with a stable course of hallucinations decreased with increasing severity from 63% at NPI ≥1 to 36% at NPI ≥4 (see Supplementary Table 1 for more details). Forty-seven (55%) patients with LBD had significant delusions, half of which were single episodes, whereas relapsing course occurred in only seven. Unlike Alzheimer's disease, delusions were not associated with severe total NPS in LBD.
The most severe patient's course
Patients with Alzheimer's disease with the highest NPI total scores (22 patients, mean NPI total 38, s.d. 12, 163 observations) had more stable (55%) and relapsing (43%) course of four key symptoms: aberrant motor behaviour (wandering), aggression/agitation, delusions and irritability (abbreviated WADI). This was not seen in LBD to the same degree. The association between having all WADI symptoms stable or relapsing with high NPI total scores was high, with an odds ratio of 55 (s.d. 12.7–248.7, P < 0.001). Aberrant motor behaviour reoccurred in all 22 patients; delusions were present in 21 and reoccurring in 19. Generally for all patients, severe aggression (item score ≥8) reoccurred in 11 of 32 patients with Alzheimer's disease and severe aggression, but only two of 18 patients with LBD showed reoccuring severe aggression (item score ≥8, P = 0.001, Supplementary Table 1). Similarly, patients with Alzheimer's disease were more likely to have a stable course of irritability (Table 3, P = 0.003).
Discussion
We studied the individual course of NPS in a cohort of patients with LBD and Alzheimer's disease for up to 12 years. There were wide variations between patients, diagnoses and specific NPS. Nearly all patients had clinically significant NPS; single episodes represented the most common course, followed by a relapsing course, whereas a stable course was less common.
The finding that single and relapsing courses are common is in line with some earlier studies, but we found less stable symptoms than most comparable studies.Reference Hope, Keene, Fairburn, Jacoby and McShane6,Reference Ballard, O'Brien, Swann, Thompson, Neill and McKeith21 Methodological differences such as selection criteria, psychometric instruments, frequency of assessments and the duration of study make comparisons between studies difficult, but a more relapsing pattern of affective symptoms (not including apathy) is similar.Reference Hope, Keene, Fairburn, Jacoby and McShane6 Few studies have assessed the long-term course of psychotic symptoms, but they are reported as either persistent or single episodes;Reference Ballard, Margallo-Lana, Fossey, Reichelt, Myint and Potkins22 studies also report greater stability for hallucinations compared with delusions.Reference van der Linde, Dening, Stephan, Prina, Evans and Brayne1,Reference Hope, Keene, Fairburn, Jacoby and McShane6,Reference Ballard, O'Brien, Swann, Thompson, Neill and McKeith21 The lower stability of hallucinations seen in our cohort may be due to a longer follow-up and longer intervals between assessments. Differences between delusions and hallucinations in Alzheimer's disease are in line with genetic findings of delusions but not hallucinations being associated with schizophrenia risk genes.Reference Creese, Vassos, Bergh, Athanasiu, Johar and Rongve23 Several studies show that cross-sectional NPI assessments can be statistically reduced to subsyndromes such as psychosis, hyperactivity and affective symptoms, using principal component analysis or factor analysis. The NPI was not originally designed for this.Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein16 Longitudinal data have challenged the usefulness of such subsyndromesReference Haaksma, Leoutsakos, Bremer, Aalten, Ramakers and Verhey4,Reference Connors, Seeher, Crawford, Ames, Woodward and Brodaty24 because of the assumption that the NPI item scores are continuous and the data inflation of scores equal to 0. In line with this, we have shown a high degree of instability of symptoms when assessed longitudinally. The instability and the differences between assumed associated symptoms, like hallucination and delusions in recent studies, argue against clearly defined subsyndromes of NPS, and underline the importance of full psychiatric assessments in clinical practice and trials.
Interestingly, the reported association between WADI symptoms and high NPI total score included delusions but not hallucinations; we did not find a similar pattern for LBD. Other single symptoms studies report of wandering or aggressive resistance persistent over 1–2 years, but no study has reported such a long course or association between WADI symptoms and severe NPS. An effort to identify the subgroups of patients with Alzheimer's disease with persistent and severe NPS may improve both ordinary treatment and trials. Atrophy of the prefrontal cortex has been reported to be associated with the stability of NPS over 6 months, whereas amyloid angiopathy is associated with early and severe psychotic symptoms in diagnosed Alzheimer's disease.Reference Poulin, Bergeron and Dickerson25,Reference Vik-Mo, Bencze, Ballard, Hortobagyi and Aarsland26
Few studies have analysed the course of symptoms in LBD compared with Alzheimer's disease, but a 1-year study found similar differences in hallucinations.Reference Ballard, Bannister, Graham, Oyebode and Wilcock27 The differences between Alzheimer's disease and LBD in depression and aggression are in line with studies with shorter duration.Reference Kazui, Yoshiyama, Kanemoto, Suzuki, Sato and Hashimoto28 We included PDD in LBD, which could bias our results, but PDD is reported to have an NPS profile more like Alzheimer's disease than DLB.Reference Chiu, Tsai, Chen, Chen and Lai29 We have previously shown that DLB is only associated with more frequent hallucination, delusions and apathy when controlling for the rate of cognitive decline and time.Reference Vik-Mo, Giil, Ballard and Aarsland5 Current data show that only hallucinations have more stable course in DLB, whereas anxiety, irritability and aberrant motor behaviour are less stable in DLB compared with Alzheimer's disease, but this may also be biased by shorter survival in the DLB group, which also had a slightly longer reported duration of symptoms before inclusion.
Important for dementia carers are the highly individual course of NPS, demonstrating the importance of personalised medicine. A majority of patients also experienced relapsing psychotic symptoms. These findings are important for clinicians because they suggest that although psychosocial strategies with low risk for adverse effects should always be considered for NPS first, they may also be unnecessary, particularly for more costly interventions of longer duration. The administration of antipsychotics or other psychotropic drugs with limited effect and low tolerability might not be necessary because NPS often tend to remit spontaneously. These findings are also important for the planning of trials for NPS, showing that spontaneous resolution is common and thus reducing the chances of finding significant drug effects.
Strengths and limitations
The strengths of the study include the long follow-up time and the very high completeness of data, except for attrition owing to death, which is a unique feature of this study. Patients were included at the time of diagnosis and most of them were followed until death. Unlike most previous studies, we report separately for Alzheimer's disease and LBD, with a systematic diagnostic evaluation during the study period and autopsy confirmation of a subgroup.
Limitations include the potential for referral bias because recruitment happened at specialist clinics, but general practitioners were invited to refer any patients with suspected dementia, and patients were included from psychiatric, neurologic and geriatric clinics. We did not have full clinical description of the excluded and non-consenting patients. The interval between assessments was 1 year, and thus we do not know what happened between assessments. The informant to NPI (carer or family) changed over follow-up for most patients. Finally, we did not standardise drug and psychosocial management, which might have influenced the course. The restrictive use of psychotropics in the Demvest study is consistent with the most recent guidelines.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.195.
Funding
This work was supported by government through Western Norway Regional Health Authority (Helse Vest including Helse Stavanger) and The Norwegian Health Association (non-profit organisation).
Acknowledgements
We acknowledge the dedicated work of research staff in the Demvest study and Helse Vest. This paper represents independent research partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.







eLetters
No eLetters have been published for this article.