Depression causes a high global disease burden.Reference Üstü, Ayuso-Mateos, Chatterji, Mathers and Murray1 In Europe, the total annual cost of depression was estimated at €118 billion in 2004.Reference Sobocki, Jonsson, Angst and Rehnberg2 The majority of patients with depression are treated in primary care settings.Reference Thielke, Vannoy and Unutzer3 A large amount of evidence shows that collaborative care in these settings is effective in reducing depression symptoms,Reference Gilbody, Bower, Fletcher, Richards and Sutton4 while also being cost-effective.Reference van Steenbergen-Weijenburg, van der Feltz-Cornelis, Horn, van Marwijk, Beekman and Rutten5 In Germany, more than 50% of primary care practices are solo physician practices with limited resources,6,Reference Bodenheimer and Laing7 where extensive collaborative models would be difficult to implement. Primary care practices located in rural areas often have limited access to mental health specialists. In view of these challenges, non-medical healthcare professionals may be a cost-effective resource for depression management in typical primary care settings.Reference Bodenheimer and Laing7 Rost et al showed that nurse assistants can successfully take part in multifaceted depression quality improvement programmes in a primary care setting.Reference Rost, Nutting, Smith, Werner and Duan8 Hunkeler et al trained nurse assistants in a depression management programme from an integrated health maintenance organisation.Reference Hunkeler, Meresman, Hargreaves, Fireman, Berman and Kirsch9 Klinkman et al showed the long-term effects of a multifaceted intervention for patients with chronic depression.Reference Klinkman, Bauroth, Fedewa, Kerber, Kuebler and Adman10 In Germany, healthcare assistants are not as well-qualified as physician assistants or nurse assistants,Reference Bosley and Dale11 since they only receive 2 years of basic vocational training (1 day of lectures per week). They generally perform administrative tasks and basic medical care, and work in primary care settings. A healthcare assistant can earn between €19 400/year (3 years of professional experience) and €32 000/year (more than 30 years of experience).12 We have already shown that depression case management provided by healthcare assistants in small, private primary care practices over a 12-month period is effective in improving symptoms and the process of care among patients with major depressionReference Gensichen, von Korff, Muth, Peitz, Beyer and Guethlin13 and here we evaluate the cost-effectiveness of this intervention.
Method
Study design and participants
We designed a pragmatic cluster-randomised controlled trial with the primary care practice as the unit of randomisation in order to avoid potential contamination (trail registration: ISRCTN66386086). The study took place in central Germany between 2005 and 2008. We assessed patients at baseline, and after 6, 12 and 24 months. The intervention lasted 12 months (between baseline and the 12-month assessment). Details on the methods employed in the trial have been published elsewhere.Reference Gensichen, von Korff, Muth, Peitz, Beyer and Guethlin13 The institutional review board of Goethe University Frankfurt am Main, Germany, approved the study protocol on 25 April 2005. We used written consent procedures for general practitioners (GPs) and patients.
Intervention
We designed our case management intervention in accordance with the chronic care model, which emphasises proactive support for the patient by the entire practice team.Reference Wagner, Austin, Davis, Hindmarsh, Schaefer and Bonomi14 Primary care practice-based healthcare assistants, trained as depression case managers during a 2-day workshop, contacted their patients by telephone once a month for 1 year. They monitored symptoms of depression using a structured questionnaire and supported medication adherence. They also encouraged patients to undertake pleasant activities. The assistants then reported the results of the call to the GP in a structured manner, stratifying the urgency of contact in terms of symptom severity. This intervention was provided in addition to usual care. Patients in the control group received usual care.
Data collection
At each study assessment, patients completed the Patient Health Questionnaire-9 (PHQ-9),Reference Gensichen, von Korff, Muth, Peitz, Beyer and Guethlin13 a 9-item questionnaire on depressive symptoms, as well as the EQ-5D.15 The EQ-5D is a simple questionnaire for the subjective description of perceived state of health. It also provides a preference-based utility score (EQ-5D index) for each of the EQ-5D health states with the best state (perfect health) and ‘death’ being assigned values of 1 and 0, respectively.Reference Dolan16 In addition, patients completed a questionnaire on healthcare utilisation (including psychiatric in-patient care, out-patient care provided by psychologists, psychiatrists and family doctors/GPs as well as antidepressant drug use) and lost work days in the 12 months preceding the interview. Additional utilisation data were collected from the patients' medical records. Thirteen intervention practices were randomly selected to assess intervention costs using a structured questionnaire.
Data analysis
Following the concept of cost-utility analysisReference Drummond, Sculpher, Torrance, O'Brien and Stoddart17 we calculated quality-adjusted life-years (QALYs) as the primary outcome to measure health effects over 24 months. To calculate QALYs we used health state utilities based on the EQ-5D index provided by Dolan.Reference Dolan16 Depression-free days (DFDs) were calculated for our secondary outcome, using the method of Lave et al:Reference Lave, Frank, Schulberg and Kamlet18 if patients had a PHQ-9 score of ⩾15, they were assumed to have ‘full’ depression (100% depression or 0% DFDs); when scoring ⩽4 they were assumed to have no depression (0% depression or 100% DFDs); if they scored between 4 and 15, the DFDs were weighted proportionately using linear interpolation. The QALYs (DFDs) were calculated by multiplying the arithmetic average of EQ-5D index scores (DFD scores) from two neighbouring measurement points by the time period between these measurement points; in other words, we calculated the area under the curve of linearly interpolated EQ-5D index scores (DFD scores) over time. To calculate direct healthcare costs from a societal perspective, we assigned monetary values to patient care according to a German guidelineReference Krauth, Hessel, Hansmeier, Wasem, Seitz and Schweikert19 and adjusted for inflation based on the year 2006. Using our measure, the costs of a visit to a primary care physician was €17.28, to a psychiatrist €29.65, a psychotherapist session was €50.29 and a psychiatric in-patient hospital day cost €240.31. The costs of prescribed antidepressant drugs were based on defined daily doses using unit costs from a national drug database.20 Almost all intervention costs resulted from time being spent by primary care physicians (€54.42 per hour) and healthcare assistants/case managers (€18.34 per hour).21 Indirect costs due to lost work days of the patients were valued at €90.67 per day21 following the human capital approach. The price year used was 2006. We did not apply discounting since the study's follow-up period was only 2 years. Discounting costs and effects hardly affected the results. The incremental cost-effectiveness ratio (ICER) was calculated as the ratio of differences in mean costs and mean number of QALYs or DFDs, respectively, between the intervention group and the control group at the 24-month follow-up. Differences in means were analysed using non-parametric bootstrapping (4000 replications) to take into account the skewed distribution of cost and effectiveness data. To visualise the statistical uncertainty of the ICER, cost-effectiveness acceptability curves were constructed using a net-benefit regression approach with bootstrap corrected standard errors (1000 replications).Reference Hoch, Rockx and Krahn22
Data analysis was based on patients for whom information was available from baseline and at least one follow-up assessment. Within this sample, missing values were replaced using the last-observation-carried-forward method. The rate of missing values on the EQ-5D varied between 1 and 6%. On the PHQ-9 the missing value rate varied between 0.3 and 5% and tended to be higher in the intervention group during the follow-up. We used LOCF for both costs and outcomes. Data analysis was performed with the Stata software package (Release 10) for Windows, applying an α = 0.05 level of significance.
Results
We enrolled 74 primary care practices and 626 patients at baseline (310 in the intervention group and 316 in the control group). Most primary care practices were run by only one or two GPs. After 24 months, 439 (70.1%) patients (209 in the intervention group and 230 in the control group) in 71 practices could be assessed. For 562 patients (268 in the intervention group, 294 in the control group) data were available from baseline and at least one follow-up assessment. This sample was used for the analysis (Table 1).
Depression outcome and utilities
There was no significant difference in EQ-5D index scores, and thus QALYs, between the intervention and the control group. But at the 24-month follow-up, the intervention group had experienced a statistically significant, higher number of DFDs than the control group (373 v. 311, P<0.01). The mean PHQ-9 scores of the intervention group were lower after 6 months (11.9 v. 13.2, P = 0.007) and 12 months (10.7 v. 12.1, P = 0.009). After 24 months a difference between the groups was still apparent, but was not statistically significant (10.5 v. 11.5, P = 0.085).
Resource use and costs
During the 24-month follow-up period patients in the intervention group tended to spend more days in psychiatric in-patient care and missed fewer work days than patients in the control group (Table 2). The annual intervention costs were €276 per patient (Table 3), as reported elsewhere.Reference Baron, Heider, Gensichen, Petersen, Gerlach and Krauth23 During the 24-month follow-up period there was no statistically significant difference between the two groups with regard to the mean direct costs (€4495 v. €3506, P = 0.16), nor with regard to the various categories of direct costs (Table 3). Mean indirect costs were considerably lower in the intervention group (€5228 v. €7539, P = 0.06), resulting in lower mean total (direct + indirect) costs (€9723 v. €11 045, P = 0.41). Yet these differences were not statistically significant either.
Cost-effectiveness
The point estimate for the cost-utility ratio was €38 489 per QALY gained (which corresponds to £31 127 per QALY gained on the basis of an exchange rate on 31 December 2006 of €1 = £0.81) if only direct costs were considered, and the ‘dominance’ of the intervention if total costs were considered (Table 3). Yet, regardless of decision makers' willingness to pay per QALY, the probability of the intervention being cost-effective was never above 90% (Fig. 1). When only direct costs were taken into account, the point estimate for the ICER of the intervention was €16 per DFD. When total costs were considered, the intervention was less costly, resulting in the ‘dominance’ of the intervention (i.e. it being less costly and more effective). If decision makers were willing to pay €10, €50 or €100 per DFD, the respective probabilities of the intervention being cost-effective would be 31.1%, 93.3% or 98.3% (when only direct costs were considered), and 88.7%, 98.4% or 99.5% (when total costs were taken into account). We estimated a 90% probability that the intervention would be cost-effective, if decision makers were willing to pay €12 per DFD.
Table 1 Practice and patient characteristics at baseline
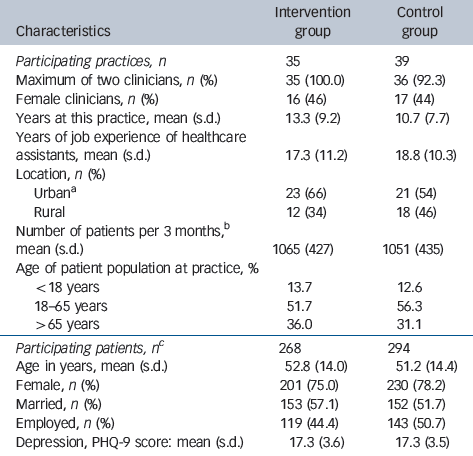
| Characteristics | Intervention group | Control group |
|---|---|---|
| Participating practices, n | 35 | 39 |
| Maximum of two clinicians, n (%) | 35 (100.0) | 36 (92.3) |
| Female clinicians, n (%) | 16 (46) | 17 (44) |
| Years at this practice, mean (s.d.) | 13.3 (9.2) | 10.7 (7.7) |
| Years of job experience of healthcare assistants, mean (s.d.) | 17.3 (11.2) | 18.8 (10.3) |
| Location, n (%) | ||
| UrbanFootnote a | 23 (66) | 21 (54) |
| Rural | 12 (34) | 18 (46) |
| Number of patients per 3 months,Footnote b mean (s.d.) | 1065 (427) | 1051 (435) |
| Age of patient population at practice, % | ||
| <18 years | 13.7 | 12.6 |
| 18-65 years | 51.7 | 56.3 |
| >65 years | 36.0 | 31.1 |
| Participating patients, n Footnote c | 268 | 294 |
| Age in years, mean (s.d.) | 52.8 (14.0) | 51.2 (14.4) |
| Female, n (%) | 201 (75.0) | 230 (78.2) |
| Married, n (%) | 153 (57.1) | 152 (51.7) |
| Employed, n (%) | 119 (44.4) | 143 (50.7) |
| Depression, PHQ-9 score: mean (s.d.) | 17.3 (3.6) | 17.3 (3.5) |
a. Refers to a town with >50 000 inhabitants.
b. In Germany, panel size is given as the number of patient registrations in a practice in 3 months.
c. Refers to participants (n = 562) for whom information was available at baseline and at least 1 follow-up assessment.
Table 2 Mean resource use and work-loss days during 24-month follow-up
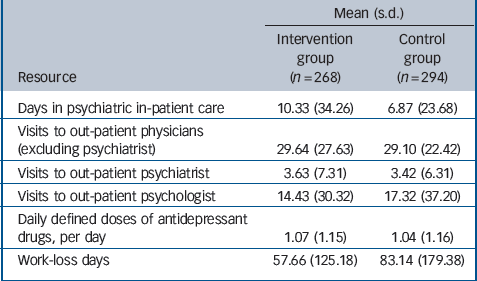
| Mean (s.d.) | ||
|---|---|---|
| Resource | Intervention group (n = 268) | Control group (n = 294) |
| Days in psychiatric in-patient care | 10.33 (34.26) | 6.87 (23.68) |
| Visits to out-patient physicians (excluding psychiatrist) | 29.64 (27.63) | 29.10 (22.42) |
| Visits to out-patient psychiatrist | 3.63 (7.31) | 3.42 (6.31) |
| Visits to out-patient psychologist | 14.43 (30.32) | 17.32 (37.20) |
| Daily defined doses of antidepressant drugs, per day | 1.07 (1.15) | 1.04 (1.16) |
| Work-loss days | 57.66 (125.18) | 83.14 (179.38) |
Table 3 Mean costs, mean number of depression-free days and incremental cost-effectiveness ratio (ICER) during 24-month follow-upFootnote a
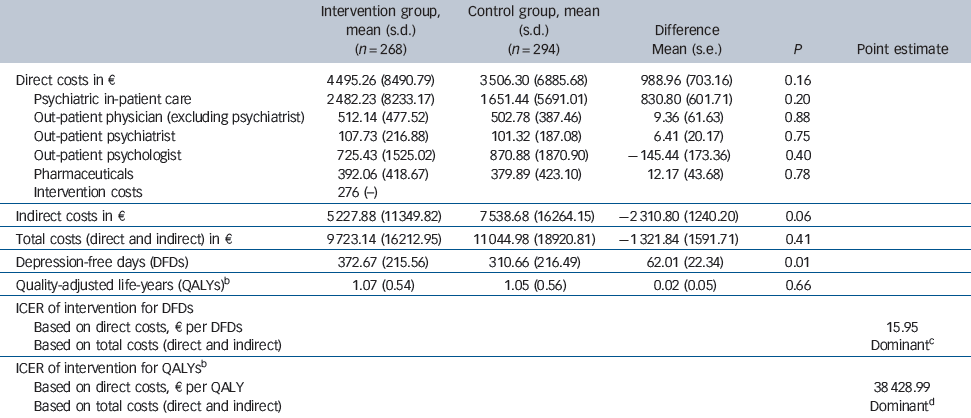
| Intervention group, mean (s.d.) (n = 268) | Control group, mean (s.d.) (n = 294) | Difference Mean (s.e.) | P | Point estimate | |
|---|---|---|---|---|---|
| Direct costs in € | 4 495.26 (8490.79) | 3 506.30 (6885.68) | 988.96 (703.16) | 0.16 | |
| Psychiatric in-patient care | 2 482.23 (8233.17) | 1 651.44 (5691.01) | 830.80 (601.71) | 0.20 | |
| Out-patient physician (excluding psychiatrist) | 512.14 (477.52) | 502.78 (387.46) | 9.36 (61.63) | 0.88 | |
| Out-patient psychiatrist | 107.73 (216.88) | 101.32 (187.08) | 6.41 (20.17) | 0.75 | |
| Out-patient psychologist | 725.43 (1525.02) | 870.88 (1870.90) | –145.44 (173.36) | 0.40 | |
| Pharmaceuticals | 392.06 (418.67) | 379.89 (423.10) | 12.17 (43.68) | 0.78 | |
| Intervention costs | 276 (−) | ||||
| Indirect costs in € | 5 227.88 (11349.82) | 7 538.68 (16264.15) | –2 310.80 (1240.20) | 0.06 | |
| Total costs (direct and indirect) in € | 9 723.14 (16212.95) | 11 044.98 (18920.81) | –1 321.84 (1591.71) | 0.41 | |
| Depression-free days (DFDs) | 372.67 (215.56) | 310.66 (216.49) | 62.01 (22.34) | 0.01 | |
| Quality-adjusted life-years (QALYs)Footnote b | 1.07 (0.54) | 1.05 (0.56) | 0.02 (0.05) | 0.66 | |
| ICER of intervention for DFDs | |||||
| Based on direct costs, e per DFDs | 15.95 | ||||
| Based on total costs (direct and indirect) | DominantFootnote c | ||||
| ICER of intervention for QALYsFootnote b | |||||
| Based on direct costs, € per QALY | 38 428.99 | ||||
| Based on total costs (direct and indirect) | DominantFootnote d |
a. Standard error (s.e.) and P for test of difference in means between intervention and control group are based on non-parametric bootstrapping with 4000 replications taking into account clusters.
b. Based on n = 255 observations in intervention group and n = 278 observations in control group due to missing values for EQ-5D index (QALYs).
c. The intervention was associated with lower mean costs and higher mean depression-free days.
d. The intervention was associated with lower mean costs and more QALYs.
Discussion
Although most research has examined academic or integrated settings as in health maintenance organisations,Reference Williams, Gerrity, Holsinger, Dobscha, Gaynes and Dietrich24 this study highlights the benefits of a low-intensity intervention in small, private primary care practices with limited resources. This trial indicates that the involvement of healthcare assistants in the care of patients with depression may be a cost-effective resource for small primary care settings. This is noteworthy, since the need for cost-effective healthcare interventions in primary care settings is increasing, and healthcare assistants (who have limited training) are employed in the majority of small primary care practices.
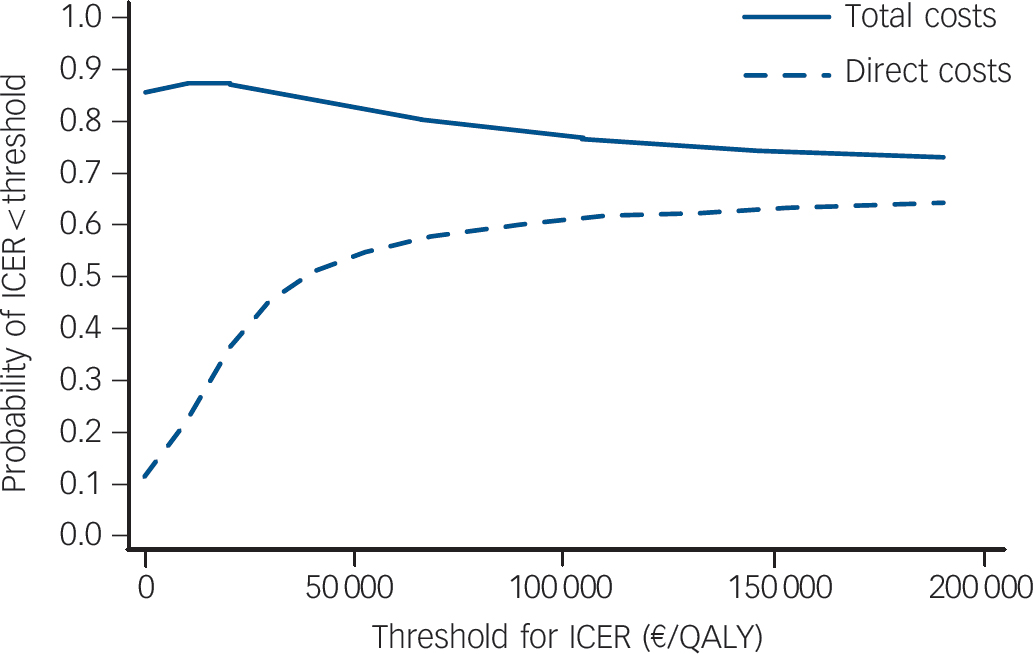
Fig. 1 Cost-effectiveness acceptability curves for direct and total costs.
Underlying regression models were bootstrapped with 1000 replications. Cluster structure of the data was taken into account. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-years.
Comparison with findings from other studies
Comparison of our results with cost-effectiveness studies of other - mostly more complex - collaborative care interventions for depression conducted in the USAReference Katon, Schoenbaum, Fan, Callahan, Williams and Hunkeler25-Reference Pyne, Fortney, Tripathi, Maciejewski, Edlund and Williams33 is limited because of differences in healthcare systems and included cost categories. However, it is noteworthy that our rather simple model is the first collaborative care intervention in which dominance over usual care could be shown with regard to both costs per DFD and costs per QALY. This is mainly because of the reduction in indirect costs, which has not been included in any of the existing studies. Indirect costs are mainly attributable to lost work days, which is a relevant measure for working patients (about two-thirds in our study sample). Because of the close agreement with claims data that can be shown for the collection of data on resource utilisation by patients with mental illness via telephone interview, we assume that the collection of lost work days by patients with mental illness via personal interviews should be of sufficient quality.Reference Heinrich, Deister, Birker, Hierholzer, Weigelt and Zeichner34
When our cost data were converted to US dollar purchasing power parities and those of other studies are de-/inflated to the year 2006 to facilitate comparison, we found two other studies reporting lower direct costs per DFD than our study. Katon et al Reference Katon, Schoenbaum, Fan, Callahan, Williams and Hunkeler25 evaluated the IMPACT intervention, which focused on elderly patients and is based on a case manager who follows up the patient by telephone, coordinates antidepressant therapy and performs a 6- to 8-session psychotherapy programme, if necessary. Liu et al Reference Liu, Hedrick, Chaney, Heagerty, Felker and Hasenberg26 evaluated a team-based intervention incorporating a clinical psychologist, a psychiatrist, a social worker and a psychology technician. The intervention comprised diagnosis, treatment, patient education, patient support and progress evaluation. The more favourable ICER of these more complex interventions was as a result of greater effects (gain of 107 DFDs over 24 months) in the case of Katon et al and very low incremental costs for a gain of 15 DFDs over 9 months in the case of Liu et al. Direct costs per DFD similar to our study were reported by Rost et al Reference Rost, Pyne, Dickinson and LoSasso27 who evaluated a management programme focusing on the encouragement of patients to follow a guideline-concordant therapy and on its maintenance, and by Simon et al Reference Simon, Von Korff, Ludman, Katon, Rutter and Unutzer28 who analysed a relapse prevention programme consisting of patient education and frequent visits, telephone and mail contacts. Rost et al showed a gain of 59 DFDs over 24 months whereas Simon et al found a gain of 14 DFDs over 12 months. The direct costs per DFD reported by three further studiesReference Simon, Katon, VonKorff, Unutzer, Lin and Walker29-Reference Schoenbaum, Unutzer, Sherbourne, Duan, Rubenstein and Miranda31 were nearly twice as high as in our study.
Direct costs per QALY have been reported for six collaborative care interventions. Three of these interventions lead to more favourable ICERs than in our study,Reference Katon, Schoenbaum, Fan, Callahan, Williams and Hunkeler25,Reference Rost, Pyne, Dickinson and LoSasso27,Reference Pyne, Rost, Zhang, Williams, Smith and Fortney32 one had an ICER comparable with our results,Reference Schoenbaum, Unutzer, Sherbourne, Duan, Rubenstein and Miranda31 and two had less favourable ICERs.Reference Schoenbaum, Unutzer, Sherbourne, Duan, Rubenstein and Miranda31,Reference Pyne, Fortney, Tripathi, Maciejewski, Edlund and Williams33 The studies showing more favourable ICERs reported a QALY gain more than twice as high as found in our study.
Using DFDs as a measure
With regard to the difference of 62 DFDs between the groups over 24 months, we did not expect larger effects since the intervention was in addition to regular primary care and, unlike other trials, did not make use of expensive psychiatrist or psychologist contacts.Reference Simon, Katon, Lin, Rutter, Manning and Von Korff35 Case management seemed to improve the symptoms of depression during its 12 months' duration. One year after the end of the case management intervention, the effect was still apparent, but was not statistically significant. From a clinical point of view it may therefore be beneficial to the patient to extend the duration of this low-intensity, low-cost intervention, as has recently been suggested.Reference Klinkman, Bauroth, Fedewa, Kerber, Kuebler and Adman10
Depression-free days represent a patient-centred approach to measurement. Patients may benefit from DFDs, since they represent additional time for pleasant and work-related activities.Reference Ludman, Katon, Bush, Rutter, Lin and Simon36 However, decision makers may assess the value of depression interventions by direct and indirect costs as well as generic outcomes such as QALYs. Naturally, health state utilities based on the generic EQ-5D quality of life instrument capture different aspects of well-being than disease-specific DFDs based on the PHQ-9. Whereas DFDs focus on depression, depression is directly addressed by only one out of five dimensions of the EQ-5D, with the other dimensions measuring problems in other domains of quality of life. As a consequence DFDs might be more responsive to changes in depressive symptoms. This might explain why the ICER based on DFDs may appear more favourable than that based on QALYs. However, it should be pointed out that there is no threshold value for acceptable cost per DFD.
Pyne et al Reference Pyne, Tripathi, Williams and Fortney37 undertook an attempt to translate DFDs into QALYs. According to the formula developed by Pyne et al the 373 DFDs in the intervention group and the 311 DFDs in the control group would translate into 1.01 and 0.94 QALYs, respectively. This would result in a difference of 0.07 QALYS between the two groups and hence a much more favourable ICER.
Limitations
Our study has several limitations. One limitation is that direct costs were restricted to the costs of psychiatric care whereas the cost of somatic care can also be influenced by depression. In addition, costs were calculated based on self-reported data of service use and work loss days, as has been done in many other cost-effectiveness analyses. Recall bias is likely to lead to an underestimation of costs. However, as both the intervention group and the control group were possibly affected by this bias, its effect on the ICER was probably small as the ICER was calculated from differences between the two groups. Moreover, due to financial constraints in the funding of the study we could not assess intervention costs in all practices.
Another limitation is the loss to follow-up over the 24-month period, which may have led to patient selection bias. However, previously calculated sensitivity analyses have shown that the effects of the intervention on the main outcome remained statistically significant and stable under unfavourable assumptions with regard to non-participation in follow-up assessments.Reference Gensichen, von Korff, Muth, Peitz, Beyer and Guethlin13 The use of LOCF for both costs and effects is likely to result in a conservative estimate. This is caused by patients in the interventional group tending to withdraw earlier and more frequently. A complete-case analysis of our data (intervention group: n = 198, control group: n = 216) resulted in larger treatment effects than those from LOCF (DFDs: 66.22 v. 62.01; QALYs: 0.04 v. 0.02), which supports our assumption. It also holds for the difference in the total costs between both study groups (€–1931 v. €–1322).
Implications
The results of this study suggest that in small primary care practices 1 year of case management may be cost-effective. After 24 months, patients who had received case management had 62 additional DFDs, although the DFD-gain did not significantly show up in a QALY gain. Since the crucial clinical effect seems to occur during the intervention period, it may be beneficial if patients receive this low-cost intervention for longer than 12 months. The active involvement of practice-based healthcare assistants in patient care may improve depression care at economically justifiable costs.
Funding
Exclusive public funding for the Primary care Monitoring for depressive Patients Trial (PRoMPT) came from the German Ministry of Education and Research (numbers: 01GK0302 and 01GK0702). The Study was awarded the Dr. Lothar Beyer Award/German Primary Care Research Award 2008.







eLetters
No eLetters have been published for this article.