Introduction
The rainforests of Mexico are biologically productive and diverse ecosystems (Challenger, Reference Challenger1998) but are being transformed by human activity at an increasing rate. For example, the annual deforestation rate during 1990-2000 in north-eastern Chiapas, near the Palenque National Park, was 12.4%, in central Chiapas 4.5%, and on the southern Yucatan Peninsula 7.7% (Estrada et al., Reference Estrada, Luecke, Van Belle, Barreta and Rosales-Meda2004; Cayuela, Reference Cayuela2006; Serio-Silva et al., Reference Serio-Silva, Rico-Gray, Ramos-Fernández, Estrada, Garber, Pavelka and Leucke2006). Deforestation has resulted in the local and regional extinction of plant and animal species, including the black howler monkey Alouatta pigra of Mesoamerica, specifically Mexico (Tabasco, Chiapas and the Yucatan Peninsula), Guatemala and Belize (Horwich, Reference Horwich1998; Pavelka, Reference Pavelka2003; Estrada et al., Reference Estrada, Luecke, Van Belle, Barreta and Rosales-Meda2004; Serio-Silva et al., Reference Serio-Silva, Rico-Gray, Ramos-Fernández, Estrada, Garber, Pavelka and Leucke2006). A. pigra is protected by the laws of each Mesoamerican country in which it occurs (Mexico: Mexican Ecological Norms, NOM-059-SEMARNAT, 2001; Guatemala: Appendix II of the wildlife Red List, CONAP 2001; Belize: Wildlife Protection Act, WPA, 1982), and is categorized as Endangered on the IUCN Red List (IUCN, 2008) and listed in Appendix I of CITES (CITES, 2009).
Recent studies of A. pigra in Chiapas have highlighted the lack of information on the conservation status of the species in protected rainforests and fragmented sites (Estrada et al., Reference Estrada, Castellanos, García, Franco, Muñoz and Ibarra2002a, Reference Estrada, Luecke, Van Belle, Barreta and Rosales-Meda2004; Van Belle & Estrada, Reference Van Belle and Estrada2005). There are only a few studies of this species in fragmented sites in Mexico: in the Palenque National Park in the state of Campeche and in Balancan, Tabasco (Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008). Only in Belize, where the species has been studied in more detail, is there information on the impact of fragmentation, both anthropogenic and natural, on the demography, ecology, behaviour and physiology of A. pigra (Ostro et al., Reference Ostro, Silver, Koontz, Horwich and Brockett2001; Horwich et al., Reference Horwich, Brockett, James and Jones2001; Marsh, Reference Marsh and Marsh2003; Pavelka, Reference Pavelka2003; Pavelka et al., Reference Pavelka, Brusselers, Nowak and Behie2003).
Studies in fragmented landscapes report high densities of A. pigra compared to the density of the species in unfragmented rainforest. This may be the result of (1) the crowding of A. pigra into small fragments (an area effect), (2) the tolerance of these animals to habitat reduction, and/or (3) flexibility in diet (Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008). Under such conditions howler monkeys face the risk of increased parasite transmission as well as an overall increase in risk to their health because they have to descend to the ground and move between forest fragments to meet their nutritional needs (Stuart et al., Reference Stuart, Greenspan, Glander and Clarke1990; Stoner, Reference Stoner1996; Ascencio et al., Reference Ascencio, Arroyo-Rodríguez, Dunn and Azkarate2009).
The main questions that we address here are: (1) What is the population size and density of A. pigra in Playas de Catazajá, Chiapas? (2) How are troops distributed and what are the age and sex composition and size of the troops? (3) Which vegetation fragments are more suitable for the conservation of A. pigra in this region?
Study area
The municipality of Playas de Catazajá is in north-east Chiapas, Mexico, at an altitude of 20 m. The site studied covers c. 621 km2 (Fig. 1). Playas de Catazajá is part of a system of wetlands in the Usumacinta River basin. The bloodwood tree Haematoxylon campechianum is typical of this region of low sub-perennial rainforest and riparian and secondary vegetation (Pennington & Sarukhán, Reference Pennington and Sarukhán1998). The remnant vegetation in Playas de Catazajá is mainly managed, secondary and riparian vegetation surrounded by a matrix of pastures.
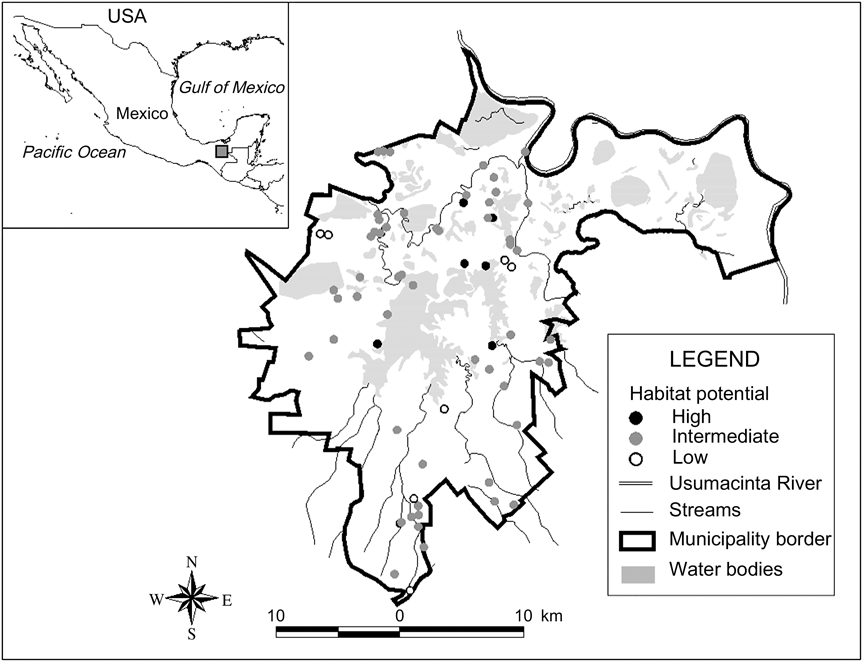
Fig. 1 The study area of Playas de Catazajá, Chiapas, illustrating the distribution of the studied forest fragments and the index of habitat potential (see text for details) for conservation of black howler monkey Alouatta pigra in Playas de Catazajá, Chiapas. The rectangle on the inset indicates the location of the main figure in Mexico.
Methods
A complete census of A. pigra was carried out in each of the 115 forest fragments in 46 ejidos (communal land for agriculture under the stewardship of rural inhabitants). We assumed that we are able to locate all A. pigra groups present in these small fragments. This was accomplished by a team of 3–4 people walking slowly to cover the entirety of each fragment; this method has been used by other authors working with Alouatta under similar conditions (Zunino et al., Reference Zunino, Kowalevsky, Oklander and Gonzalez2007; Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008). Surveys were conducted over 17 months (October 2004 to March 2006), from 06.00 to 17.00. The ejidos were randomly selected from among the 52 ejidos in Playas de Catazajá, each of which contains 1–6 forest fragments. Each ejido was surveyed on consecutive days until all chosen fragments had been censused. Ejidos with 1–3 fragments were sampled in a single day, those with 4–5 fragments in 2 days and those with six fragments in up to 3 days.
When a troop of A. pigra was sighted the animals were observed for at least 30 minutes and the age/sex composition of the troop and any other features of note (e.g. body scars) recorded. Each fragment in which A. pigra was encountered was georeferenced using a global positioning system, and the data transferred to orthophotos (1 : 20,000) using the geographical information system ArcView v. 3.2 (ESRI, Redlands, USA). Collection of detailed information on each troop ensured that we did not repeat observations on individual troops. Density was calculated as the number of individuals in the total area surveyed (691.2 ha).
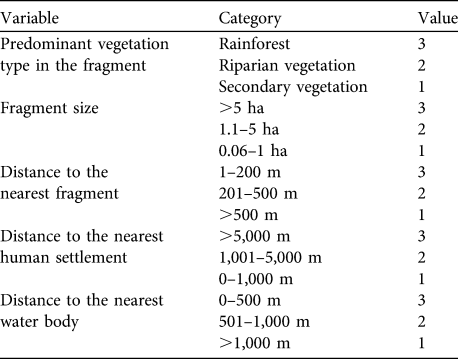
We drew distribution maps for A. pigra and identified habitats for conservation based on five factors that have been identified as important for the species in fragmented habitat (Marsh, Reference Marsh and Marsh2003; Rodríguez-Toledo et al., Reference Rodríguez-Toledo, Mandujano, García-Orduña and Marsh2003; Anzures-Dadda & Manson, Reference Anzures-Dadda and Manson2007): vegetation type (recorded for each fragment visited: rainforest, riparian vegetation, secondary vegetation) and fragment size, and distances to the nearest fragment, human settlement and water body from fragment edge. Variables other than vegetation type were calculated using ArcView.
For fragments that contained A. pigra multiple linear regression was used to evaluate the relationship of abundance and troop size with area of fragment and distances to the nearest fragment, human settlement and water body. A non-parametric one-way ANOVA (Kruskall-Wallis) was used to identify any significant differences in these characteristics between fragments with and without A. pigra.
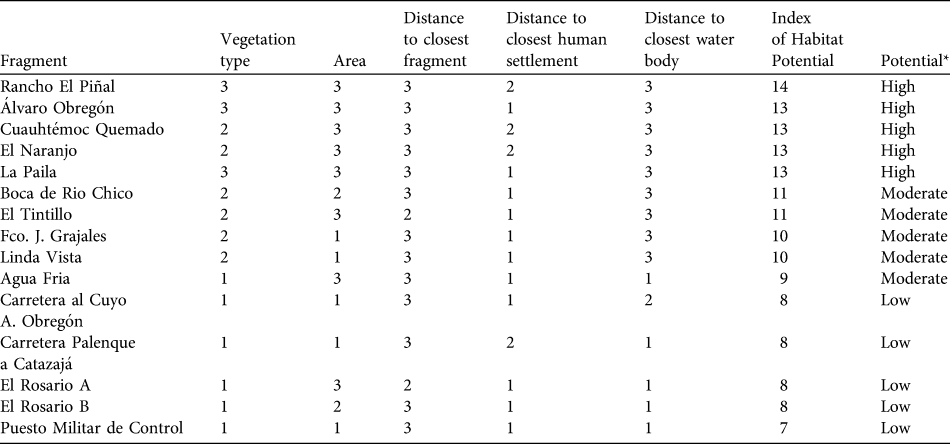
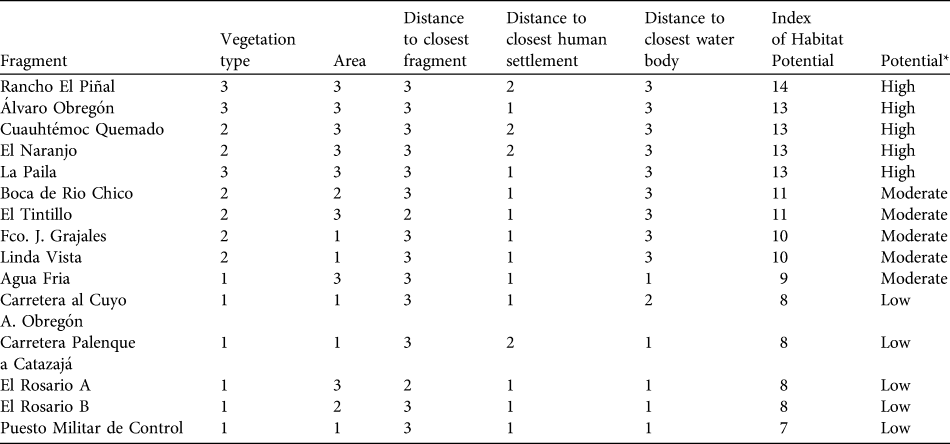
For each fragment inhabited by A. pigra we calculated an index of habitat potential (also known as the ecotourism potential index; Berovides-Álvarez, 2000) for conservation. Each independent variable was scored in the range 1–3, where 3 represents the most favourable condition of the variable for conservation of the species, 1 the least favourable and 2 an intermediate condition (Table 1). These scores are summed for the five variables, giving an index with a potential range of 3–15. Fragments with an index in the range 13–15 are considered to have high potential suitability for the conservation A. pigra, those with 9–12 moderate potential, and fragments with ≤ 8 low potential (Table 2).
Table 1 The five criteria used to generate the index of habitat potential (see text for further details) for the conservation of Alouatta pigra.
Results
A. pigra was present in 70 of the 115 vegetation fragments surveyed. We recorded 659 individuals in 118 troops, and 11 solitary individuals, giving an overall density of 95.3 km-2. Mean troop size was 5.0 ± SE 2.6 (range 2–13), and there were 435 adults, 137 juveniles and 87 infants (Table 3). The most common social unit was single male–multi-female (50 troops, 42%), followed by multi-male–multi-female (39, 33%), single male–single female (16, 14%), and single female–multi-male (10, 8%). The remaining 3% (n = 3) were same sex pairs.
Table 3 Total area, area surveyed and area in which A. pigra was present, and abundance, by age and sex, in Playas de Catazajá (Fig. 1).
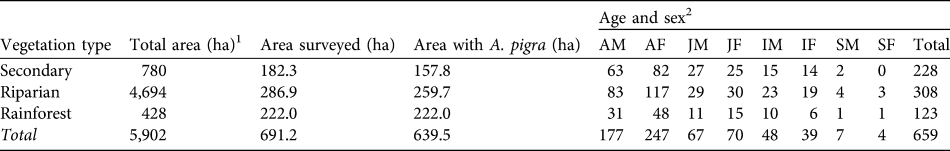
1 Total area in the municipality
2 AM, adult male; AF, adult female; JM, juvenile male; JF, juvenile female; IM, infant male; IF, infant female; SM, Solitary male; SF, solitary female
The mean number of adult males per group was 1.4 ± SE 0.7, with 2.0 ± SE 0.1 adult females, 0.6 ± SE 0.1 juvenile males, 0.6 ± SE 0.1 juvenile females, 0.4 ± SE 0.6 male infants, and 0.3 ± SE 0.04 female infants. Two troops had no adult males, one had no adult females and 13 had no immatures. The adult male to female ratio was 1 : 1.4, the adult female to juvenile ratio 1 : 0.55 and the adult female to immature ratio 1 : 0.87.
The distribution of A. pigra in the municipality of Catazajá is limited to low-lying areas that are prone to flooding, such as riparian vegetation, and rainforests with trees of low to medium height near the municipality of Palenque, Chiapas. Of the individual A. pigra recorded, 46.7% (n = 308) were sighted in areas with riparian vegetation, 34.6% (n = 228) in secondary vegetation and 18.7% (n = 123) in rainforest (Table 3).
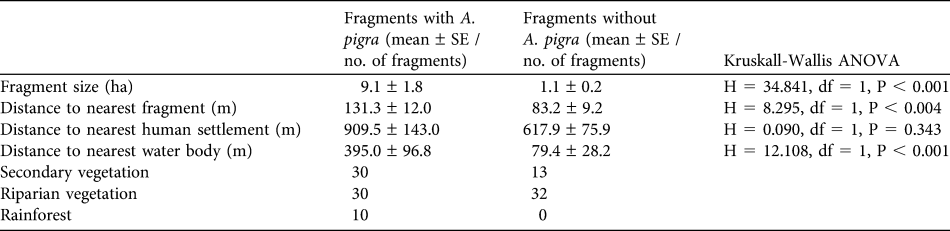
Mean fragment size was 6.01 ± SE 1.2 ha, mean distance to the nearest fragment was 112.4 ± SE 8.4 m, and mean distance to the nearest water body was 271.5 ± SE 61.5 m. Vegetation type of 53.9% of the fragments was riparian, 37.4% was secondary vegetation and only 8.7% was rainforest. The analysis between fragments with (n = 70) and without (n = 45) A. pigra revealed significant differences with respect to fragment size and distances to the nearest fragment and water body (P < 0.05; Table 4).
Table 4 For fragments in which A. pigra were and were not located, the mean of four characteristics, determined by a geographical information system (and the non-parametric Kruskall-Wallis ANOVA between the two groups of fragments), and the number of fragments of each of the three vegetation types.
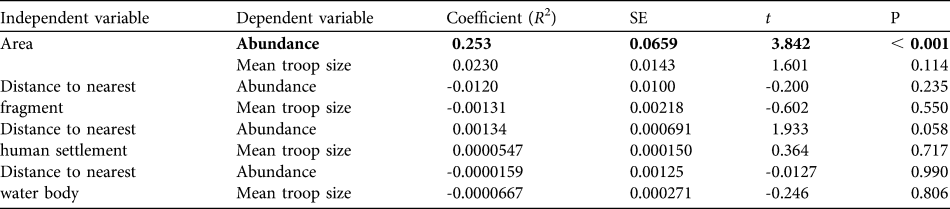
A multiple linear regression revealed no statistically significant relationship between mean troop size and fragment size and distances to the nearest fragment, human settlement or water body (R 2 = 0.04, F = 0.83, P = 0.50). A multiple linear regression of abundance and all independent variables was significant (R 2 = 0.248, F = 5.36, P ≤ 0.001) but there was a significant relationship only with fragment size (R 2 = 0.253, t = 3.842, P < 0.001; Table 5).
Table 5 Evaluation of the relationships of abundance and mean troop size of A. pigra with area of fragment and distances to the nearest fragment, nearest human settlement and nearest water body. The only significant relationship is in bold.
Of the fragments in which A. pigra was found 12.9% (n = 9; total area 2.76 km2) had a high index of habitat potential, 77.1% (n = 54; 3.43 km2) an intermediate value and 10% (n = 7; 0.20 km2) a low value (Fig. 1). Most of the fragments with low potential for conservation of A. pigra are small in area and with secondary vegetation on the edges of highways or smaller roads (Table 2).
Discussion
The density of A. pigra (95.3 km-2) in the fragments surveyed is similar to that reported for A. pigra in other fragmented sites such as around Palenque in Chiapas, Mexico (119 km-2), and the Community Baboon Sanctuary (100 km-2), Monkey River (102 km-2) and the Cockscomb Basin Wildlife Sanctuary (47–257 km-2) in Belize (Horwich & Lyon, Reference Horwich and Lyon1998; Ostro et al., Reference Ostro, Silver, Koontz, Horwich and Brockett2001; Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pavelka, Reference Pavelka2003). The highest densities reported have been attributed to the crowding of A. pigra into small areas, where they survive as a consequence of their feeding and behavioural flexibility (Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pavelka, Reference Pavelka2003; Pozo-Montuy & Serio-Silva, Reference Pozo-Montuy and Serio-Silva2007). A high density of primates in disturbed areas is likely to result in a higher parasite load and greater physiological stress than would occur in preserved areas, as well as changes in foraging strategies (Stoner, Reference Stoner1996; Martínez-Mota et al., Reference Martínez-Mota, Valdespino, Sánchez-Ramos and Serio-Silva2007; Pozo-Montuy & Serio-Silva, Reference Pozo-Montuy and Serio-Silva2007). However, some reports of high densities of A. pigra could be a result of the small number (< 30) of fragments surveyed (five fragments, Ostro et al., Reference Ostro, Silver, Koontz, Horwich and Brockett2001; 22 fragments, Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; 26 fragments, Rosales-Meda et al., Reference Rosales-Meda, Estrada and Lopez2007).
Mean troop size in Playas de Catazajá falls within the expected range for the species and did not vary with fragment size. This range is similar in fragmented and unfragmented sites (González-Kirchner, 1998; Ostro et al., Reference Ostro, Silver, Koontz, Horwich and Brockett2001; Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pavelka, Reference Pavelka2003; Van Belle & Estrada, Reference Van Belle and Estrada2005; Rosales-Meda, Reference Rosales-Meda, Estrada and Lopez2007).
There are more females than males at Playas de Catazajá, which is typical of a species with a single male–multi-female social organization. However, the number of immatures per female is low (0.8 : 1), as also reported for other fragmented sites in Mexico and Belize (immature : female = 0.9 : 1 ± SD 0.23; Horwich et al., Reference Horwich, Brockett, James and Jones2001; Ostro et al., Reference Ostro, Silver, Koontz, Horwich and Brockett2001; Estrada et al., Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2002b; Pavelka, Reference Pavelka2003; Pavelka et al., Reference Pavelka, Brusselers, Nowak and Behie2003; Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008). This suggests a decrease in reproduction compared to large areas of preserved forest such as Yaxchilán, Calakmul and Palenque National Park in Mexico and Tikal in Guatemala (immature : female 1 : 1.9 ± SD 0.22; González-Kirchner, Reference Gonzalez-Kirchner1998; Estrada et al., Reference Estrada, Luecke, Van Belle, Barreta and Rosales-Meda2004; Van Belle & Estrada, Reference Van Belle and Estrada2005). The low proportion of immatures in fragmented sites could be attributed to mortality of juveniles and infants from predation (mainly by domestic dogs when monkeys descend to the ground to feed or to move between fragments; Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008), and/or to increases in malnutrition, stress and parasitic diseases as a result of crowding (Bonilla-Moheno, Reference Bonilla-Moheno2002; Martínez-Mota, Reference Martínez-Mota2004).
Although the social unit of A. pigra has been presumed to be single male–multi-female (Horwich et al., Reference Horwich, Brockett, James and Jones2001), Van Belle & Estrada (Reference Van Belle and Estrada2005) found that social units are related to site size, with multi-male–multi-female social units observed with greater frequency in large protected areas and single male–multi-female groups in fragmented sites. The latter was the most frequent type of group in our study, and occurs with a similar frequency in fragmented sites in Palenque (Estrada et al., Reference Estrada, Castellanos, García, Franco, Muñoz and Ibarra2002a,b, Reference Estrada, Mendoza, Castellano, Pacheco, Van Belle, García and Muñoz2004; Van Belle & Estrada, Reference Estrada, Luecke, Van Belle, Barreta and Rosales-Meda2005).
The abundance of A. pigra in Playas de Catazajá is related to fragment size, as recorded at other sites (Pozo-Montuy et al., Reference Pozo-Montuy, Serio-Silva, Bonilla-Sánchez, Bynum and Landgrave2008) and for Alouatta palliata mexicana at Los Tuxtlas, Veracruz and in northern Chiapas (Estrada & Coates-Estrada, Reference Estrada and Coates-Estrada1996; Anzures-Dadda & Manson, Reference Anzures-Dadda and Manson2007). An analysis for A. palliata mexicana suggested that the risk of extinction is lowest when the area of the fragment is maintained or increased (Escobedo-Morales, Reference Escobedo-Morales2005). This indicates the need to preserve the remaining fragments by assigning them a protected status, using them only in sustainable ways, and promoting connectivity between them.
Human activities, including large-scale deforestation, agriculture and cattle ranching, are the main causes of habitat loss and fragmentation in the range of A. pigra in Playas de Catazajá. However, several other factors explain why we found the species in only 70 of the 115 fragments surveyed. The fragment size, distance to the nearest fragment and distance to the nearest water body were significantly different between fragments with and without A. pigra. Other authors have reported similar results, and attribute this to the tree composition and structural quality of the fragments for arboreal primates (Anzures-Dadda & Manson, Reference Anzures-Dadda and Manson2007).
Hunting by people for food, fire, extreme weather events and the history of habitat disturbance cause A. pigra to be absent in particular fragments on the Yucatan Peninsula and in Tabasco, Mexico (Watts & Rico-Gray, Reference Watts and Rico-Gray1987; Serio-Silva et al., Reference Serio-Silva, Rico-Gray, Ramos-Fernández, Estrada, Garber, Pavelka and Leucke2006; Pozo-Montuy & Serio-Silva, Reference Pozo-Montuy and Serio-Silva2007). We found that fragments without A. pigra were mainly in ejidos that are close to rivers and lagoons, where local people sometimes hunt A. pigra for food and the species is also more exposed to predation by domestic dogs and coyotes Canis latrans, particularly when moving on the ground to another fragment (Pozo-Montuy & Serio-Silva, Reference Pozo-Montuy and Serio-Silva2007).
The index of habitat potential is a useful tool, providing an effective instrument for making decisions and creating conservation areas, and can include any number of variables, depending on what one wishes to evaluate for particular fragments. Our data indicate that the availability of habitat is critical for A. pigra at Playas de Catazajá because only 12% of the fragments properly meet the species’ needs (i.e. areas ≥ 5 ha that are < 200 m to the nearest fragment and ≥ 1,000 m from roads and settlements). Using these data we can now guide conservation efforts for forest fragments. For example, fragment size could be increased, habitat restored, connectivity with other fragments established, and environmental education programmes could be developed and taken to the settlements near those fragments with the greatest potential for conservation of the species.
Through the establishment of environmental education programmes, local people, researchers and authorities could join together in efforts to use fragments with conservation potential as a sanctuary for A. pigra. Fragments with low to moderate conservation potential could be incorporated into a conservation plan based on community ecotourism, with A. pigra the flagship species for Playas de Catazajá. This strategy has been successfully applied elsewhere for other primate species (Horwich, Reference Horwich1998; Horwich & Lyon, Reference Horwich and Lyon1998; Goldsmith, Reference Goldsmith2001; Serio-Silva, Reference Serio-Silva2006) but prior to any implementation it will be necessary to evaluate an area’s potential for ecotourism and any threats this may represent for the species (Grossberg et al., Reference Grossberg, Treves and Naughton-Treves2003; Bonilla-Sánchez, Reference Bonilla-Sánchez2006). The study reported here has been used as supporting evidence for the classification of the Playas de Catazajá wetlands as a conservation area and its decree as a RAMSAR site at the behest of the Instituto de Historia Natural y Ecología and the government of the state of Chiapas (CONANP, 2008).
Acknowledgements
We are grateful to the inhabitants of Playas de Catazajá, Chiapas, Mexico for their invaluable assistance as field guides, Patricia Moreno, Luciana Porter and Laura T. Hernández for suggestions, and Bianca Delfosse for linguistic assistance. The Instituto de Ecología A.C. and IDESMAC A.C. provided financial and logistical support. We thank CONACYT for a scholarship (#190320) awarded to YMBS.
Biographical sketches
Yadira M. Bonilla-Sánchez is currently studying the demography, feeding ecology and behaviour of A. pigra in Eucalyptus plantations in southern Mexico. Juan Carlos Serio-Silva’s main interests are in ecology, behaviour and conservation of Mexican primates and in training a new generation of Mexican primatologists to apply their skills to increase the effectiveness of primate conservation in Mesoamerica. Gilberto Pozo-Montuy is applying conservation strategies for the protection of primates in south-eastern Mexico. His interests are in the study of ecological and conservation aspects of neotropical primates and other arboreal mammals in fragmented landscapes. Nora Bynum’s current research interests include seasonality and phenology of tropical canopy trees, particularly in relation to global change.