Article contents
Biosynthesis and Processing of Silk Proteins
Published online by Cambridge University Press: 29 November 2013
Extract
Silks produced by silkworms (e.g., Bombyx mori) and orb-web weaving spiders (e.g., Nephila clavipes) are essentially pure protein, that is, complexes of amino acid polymers. They are the most common fibers spun by biological systems. There has been a long-standing interest in the use of these and similar fibers in textiles, cables, fiber reinforcement in composites, in addition, for example, to cross hairs in optical instruments, and fishing nets. Both nylon, a homo-polymer of the amino acid glycine, and Kevlar, a polymer of a nonnatural aromatic amino acid, can be considered modified, synthetic versions of silk and are used for some of the applications mentioned above. The potential for genetic manipulation, through recombinant DNA technology, of the natural biosynthetic process for these natural proteins (see the article by Cappello in this issue) has renewed interest in the production of new silklike proteins.
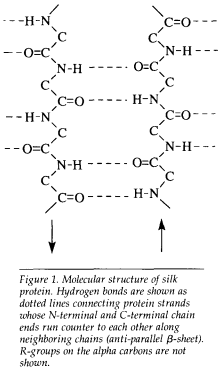
The natural silks are characterized by a β-sheet secondary structure which is stabilized by interchain hydrogen bonds and intersheet hydrophobic interactions (Figure 1). Silks can be considered block copolymers, with crystalline domains consisting of short side chain monomers (the amino acids glycine, alanine, and serine) interspersed in amorphous domains consisting of bulkier side chain amino acids.
This family of fibers is naturally tailored to perform functions such as catching prey (orb web) or serving as a barrier against environmental challenges (cocoon). The domestic silkworm (B. mori) produces only one type of silk, cocoon silk, at only one stage in its lifecyle, during the fifth larval instar just before molt to the pupa. The silk is produced in modified salivary glands and spun from the mouth.
- Type
- Biology and Materials Synthesis
- Information
- Copyright
- Copyright © Materials Research Society 1992
References
- 47
- Cited by


