Published online by Cambridge University Press: 22 October 2019
A new mineral majzlanite, ideally K2Na(ZnNa)Ca(SO4)4, was found in high-temperature exhalative mineral assemblages in the Yadovitaya fumarole, Second scoria cone of the Great Tolbachik Fissure Eruption (1975–1976), Tolbachik volcano, Kamchatka Peninsula, Russia. Majzlanite is associated closely with langbeinite and K-bearing thénardite. Majzlanite is grey with a bluish tint, has a white streak and vitreous lustre. The mineral is soluble in warm water. Majzlanite is monoclinic, C2/c, a = 16.007(2), b = 9.5239(11), c = 9.1182(10) Å, β = 94.828(7)°, V = 1385.2(3) Å3 and Z = 16. The eight strongest lines of the X-ray powder diffraction pattern are [d, Å (I, %)(hkl)]: 3.3721(40)(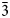 $\bar{3}$12), 3.1473(56)(
$\bar{3}$12), 3.1473(56)(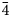 $\bar{4}$02), 3.1062(65)(
$\bar{4}$02), 3.1062(65)(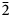 $\bar{2}$22), 2.9495(50)(
$\bar{2}$22), 2.9495(50)(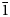 $\bar{1}$31), 2.8736(100)(
$\bar{1}$31), 2.8736(100)(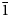 $\bar{1}$13), 2.8350(70)(421), 2.8031(45)(511) and 2.6162(41)(
$\bar{1}$13), 2.8350(70)(421), 2.8031(45)(511) and 2.6162(41)(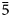 $\bar{5}$12). The following structural formula was obtained: K2Na(Zn0.88Na0.60Cu0.36Mg0.16)(Ca0.76Na0.24)(S0.98Al0.015Si0.005O4)4. The chemical composition determined by electron-microprobe analysis is (wt.%): Na2O 9.73, K2O 15.27, ZnO 11.20, CaO 7.03, CuO 4.26, MgO 1.07, Al2O3 0.47, SO3 51.34, SiO2 0.12, total 100.49. The empirical formula calculated on the basis of 16 O apfu is K1.99Na1.93Zn0.84Ca0.77Cu0.33Mg0.16(S3.94Al0.06Si0.01)O16 and the simplified formula is K2Na(Zn,Na,Cu,Mg)Σ2(Ca,Na)(SO4)4. No natural or synthetic compounds directly chemically and/or structurally related to majzlanite are known to date. The topology of the heteropolyhedral framework in majzlanite is complex. An interesting feature of the structure of majzlanite is an edge-sharing of ZnO6 octahedra with SO4 tetrahedra.
$\bar{5}$12). The following structural formula was obtained: K2Na(Zn0.88Na0.60Cu0.36Mg0.16)(Ca0.76Na0.24)(S0.98Al0.015Si0.005O4)4. The chemical composition determined by electron-microprobe analysis is (wt.%): Na2O 9.73, K2O 15.27, ZnO 11.20, CaO 7.03, CuO 4.26, MgO 1.07, Al2O3 0.47, SO3 51.34, SiO2 0.12, total 100.49. The empirical formula calculated on the basis of 16 O apfu is K1.99Na1.93Zn0.84Ca0.77Cu0.33Mg0.16(S3.94Al0.06Si0.01)O16 and the simplified formula is K2Na(Zn,Na,Cu,Mg)Σ2(Ca,Na)(SO4)4. No natural or synthetic compounds directly chemically and/or structurally related to majzlanite are known to date. The topology of the heteropolyhedral framework in majzlanite is complex. An interesting feature of the structure of majzlanite is an edge-sharing of ZnO6 octahedra with SO4 tetrahedra.
Associate Editor: Ian T. Graham