No CrossRef data available.
Article contents
Infrared reflectance of single-crystal jarandolite, CaB3O4(OH)3
Published online by Cambridge University Press: 05 July 2018
Abstract
Polarized far- and mid-infrared reflectance spectra were measured at room temperature for the new mineral species jarandolite, CaB3O4(OH)3, for the two principal directions. Thirty three vibration modes for 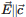 and 32 for
and 32 for 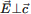 were observed and analysed numerically. Symmetry analysis predicts 41
were observed and analysed numerically. Symmetry analysis predicts 41 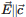 and 40
and 40 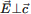 vibration modes that include lattice and O–H modes. Mode assignment was made based on the structure of jarandolite. The values of the mode frequencies (ωTO), the damping factors (γ) and the oscillator strength (S) of each oscillator were obtained by fitting to a Lorentz model.
vibration modes that include lattice and O–H modes. Mode assignment was made based on the structure of jarandolite. The values of the mode frequencies (ωTO), the damping factors (γ) and the oscillator strength (S) of each oscillator were obtained by fitting to a Lorentz model.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2007


