Published online by Cambridge University Press: 05 July 2018
The crystal structure of a Mn-bearing kilchoanite, Ca2.33Mn0.67Si2O7, orthorhombic, I2cm, a = 11.356(2), b = 5.007(1), c = 21.817(1)Å, Z = 8, was refined using single-crystal X-ray data to an R value of 3.1% for 1725 unique reflections. It is isostructural with kilchoanite, Ca3Si2O7, and shows a partial disorder of Mn and Ca over the four octahedral sites. On a basis of occupancy refinement, the M3 site (〈M3-O〉 = 2.307 Å) contains 0.508Ca and 0.492Mn, and the M4 site (〈M4-O〉 = 2.346 Å) contains 0.822Ca and 0.178Mn, whereas the M1 and M2 sites (〈M1-O〉 = 2.538 Å, (〈M2-O〉 = 2.380 Å) are occupied only by Ca. Substitution of Mn cations for Ca can be interpreted as taking place on the following sites in preferential order: (1) the smaller Ca site; (2) the less distorted Ca site; (3) the Ca site showing a loss in neutrality of formal electrostatic valence. A statistical survey of Si-O-Si angles observed in silicates containing [Si2O7] groups confirms that these angles become smaller with an increase in the coordination number of the bridging oxygen. Oversaturation of electrostatic bond-strength to the bridging oxygen in some groups of the crystal structures with two tetrahedra sharing a vertex (pyro-anion, 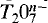 ), which is caused by increase in the coordination number of the bridging oxygen and/or by T cations with larger charge than 4+, results in longer T-Obr bonds length and in smaller T-Obr-T angles.
), which is caused by increase in the coordination number of the bridging oxygen and/or by T cations with larger charge than 4+, results in longer T-Obr bonds length and in smaller T-Obr-T angles.