Article contents
Ultrastructure of Antennal Morphology and Sensilla of Teak Skeletonizer, Eutectona machaeralis Walker (Lepidoptera: Crambidae)
Published online by Cambridge University Press: 14 October 2020
Abstract
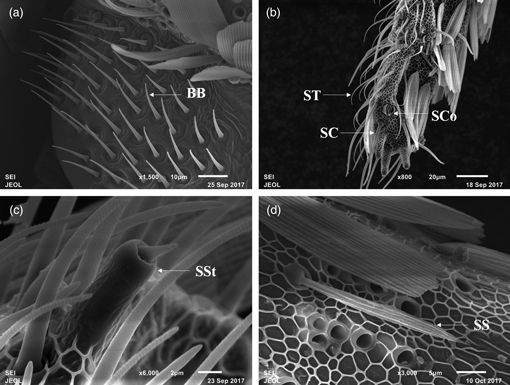
The leaf skeletonizer, Eutectona machaeralis (Walker) (Lepidoptera: Crambidae), is a severe insect pest of teak trees (Tectona grandis L.f.) in China. To provide some basic evidence for future semiochemical-based management strategies of E. machaeralis, the morphology, ultrastructure, and distribution of antennal sensilla of adults were observed under scanning and transmission electron microscopy. The shape and structure of antenna were similar between males and females, both being filiform. However, the antennal length of males was significantly longer than that of females. Eight morphological sensilla types were observed in both sexes: Böhm's bristles, sensilla trichodea, sensilla basiconica, sensilla chaetica, sensilla styloconica, sensilla coeloconica, sensilla auricillica, and sensilla squamiformia. Significant sexual dimorphism of the sensilla dimensions was found, especially in sensillar length. The putative and potential functions of the different sensilla types are discussed based on the fine structures of the cuticular walls and dendrites of the different sensilla types. We expect these results to help lay a solid foundation for future functional research and develop further investigations of E. machaeralis.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of the Microscopy Society of America
Footnotes
L-J. Lan and S-K. Wang contributed equally to this work.
References
- 5
- Cited by


