Article contents
Systematic Investigation of Lanthanoid Transition Heavy Metal Acetates as Electron Staining Reagents for Protein Molecules in Biological Transmission Electron Microscopy
Published online by Cambridge University Press: 01 April 2022
Abstract
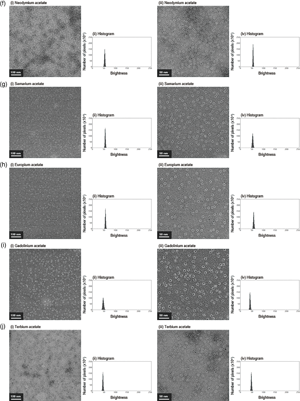
Cryo-electron microscopy, widely used for high-resolution protein structure determination, does not require staining. Yet negative staining with heavy metal salts such as uranyl acetate has been in persistent demand since the 1950s due to its image contrasting capabilities at room temperature with a common electron microscope. However, uranium compounds are nuclear fuel materials and are tightly controlled worldwide. Acetates of each lanthanoid series elements except promethium are prepared at the same concentration (2%(w/v)) and used as a model on horse spleen ferritin for electron microscopic analysis to systematically evaluate their effectiveness as electron staining reagents for the protein. Analysis shows that the triacetates of samarium and europium, followed by gadolinium and erbium, and then lanthanum and neodymium could function as electron staining reagents. Thulium-triacetate precipitates thin plate-like crystals and may be used for selecting better imaging fields. Of the 14 lanthanoid-triacetates examined, about half are viable alternatives to uranyl acetate as an electron staining reagent for ferritin, and there appears an optimal range in ionic sizes for promising lanthanoids. This is the first systematic investigation of lanthanoid transition heavy metal triacetates from the viewpoint of lanthanoid contraction, using density distribution histograms of electron micrographs as an indicator for comparison with uranyl acetate.
Keywords
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 5
- Cited by


