Article contents
Structural and Ultrastructural Changes in the Tongue of mdx Mice
Published online by Cambridge University Press: 24 January 2022
Abstract
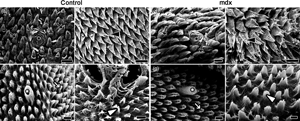
The mdx mouse is an experimental model of Duchenne muscular dystrophy, a genetic disorder characterized by progressive muscular degeneration which affects the oral cavity musculature, and promotes difficulty in swallowing. This study aimed to describe morphological, structural, and ultrastructural changes in the tongue mucosa and musculature of mdx mice. Forty six-month-old mice were divided into two groups: Control C57bl/10 (n = 20) and mdx C57bl/10mdx (n = 20). The tongue was dissected and analyzed with light microscopy, scanning electron microscopy, and transmission electron microscopy techniques. Our results showed conical and triangular filiform, fungiform, foliate, and vallate papillae, and their connective tissue cores. The epithelium layers identified were corneum, granulosum, spinosum, and basale. The mdx group had a thicker epithelium. Lamina propria was composed of reddish and greenish collagen. In mdx, collagen was present in the musculature of the tongue's body and in the muscular tissue between mucous and serous glands of the caudal region. Musculature was also characterized by a shorter length of sarcoplasmic invaginations, myocytolysis in mitochondrial groupings, and inflammatory focus. In conclusion, the tongue of 6-month-old mdx mice had morphology, structure, and ultrastructure revealed, showing higher wear of filiform papillae indirect reflex from the muscular degeneration process.
Keywords
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 1
- Cited by



