Article contents
Phase Separation in Liposomes Determined by Ergosterol and Classified Using Machine Learning
Published online by Cambridge University Press: 19 September 2022
Abstract
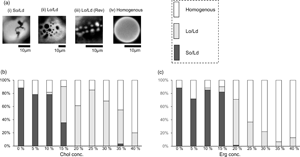
Recent studies indicated that ergosterol (Erg) helps form strongly ordered lipid domains in membranes that depend on their chemical characters. However, direct evidence of concentration-dependent interaction of Erg with lipid membranes has not been reported. We studied the Erg concentration-dependent changes in the phase behaviors of membranes using cell-sized liposomes containing 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC)/1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC). We observed the concentration range of phase separation in ternary membranes was significantly wider when Erg rather than cholesterol (Chol) was used as the sterol component. We used machine learning for the first time to analyze microscopic images of cell-sized liposomes and identify phase-separated structures. The automated method was successful in identifying homogeneous membranes but performance remained data-limited for the identification of phase separation domains characterized by more complex features.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 4
- Cited by



