Introduction
α-Crystallin in the Eye
Crystallins constitute a family of small-molecular-weight (20–30 kDa), soluble proteins found in particularly high abundance in the cytoplasm of many cells of the vertebrate optical tract. Large quantities of crystallins (20–40% of all soluble protein) were discovered in the cytoplasm of corneal cells, both in corneal epithelial cells and in stromal keratocytes (Krishnan et al., Reference Krishnan, Kathiresan, Raman, Rajini, Dhople, Aggrawal and Sharma2007; Jester, Reference Jester2008). An even greater abundance of crystallins (50–80% of all protein) was found in the cytoplasm of highly elongated fiber cells from the vertebrate lens as well as in lens epithelial cells (Delaye & Tardieu, Reference Delaye and Tardieu1983; Wistow & Piatigorsky, Reference Wistow and Piatigorsky1988; Horwitz et al., Reference Horwitz, Bova, Ding, Haley and Stewart1999; Zampighi et al., Reference Zampighi, Zampighi and Lanzavecchia2011; Andley et al., Reference Andley, Tycksen, McGlasson-Naumann and Hamilton2018).
While there are different types of crystallins (α, β, γ, and δ; Wistow & Piatigorsky, Reference Wistow and Piatigorsky1988; Wistow, Reference Wistow2012), α-crystallin is the major component of the vertebrate lens (comprising up to 45% of total lens protein), and it is the first crystallin to appear during differentiation of the murine lens (Zwaan, Reference Zwaan1983; Xia et al., Reference Xia, Wang, Tatarkova, Aerts and Clauwaert1996). α-Crystallin in the lens is a polydisperse molecular aggregate (consisting of 10–40 smaller subunits of 22–23 kDa each, with a 3:1 ratio of αA to αB subunits) that often forms a spherical particle (Horwitz et al., Reference Horwitz, Bova, Ding, Haley and Stewart1999). But recombinant crystallins can form pure αA or αB aggregates of 24 subunits each, while αA can form smaller oligomers (Peschek et al., Reference Peschek, Braun, Franzmann, Georgalis, Haslbeck, Weinkauf and Buchner2009). Sequence comparisons reveal that crystallins are members of the small heat shock protein family and form aggregates assembled from polypeptides of 10–25 kDa that share a common central domain of about 90 residues (the α-crystallin domain) with variable N- and C-terminal extensions (Augusteyn, Reference Augusteyn2004). Previously, it was suggested that, of the two subunit types that comprise multimeric α-crystallin, αA-crystallin is restricted to the lens (Chepelinsky et al., Reference Chepelinsky, King, Zelenka and Piatigorsky1985; Okazaki et al., Reference Okazaki, Yasuda, Kondoh and Okada1985; Overbeek et al., Reference Overbeek, Chepelinsky, Khillan, Piatigorsky and Westphal1985), but it was later discovered in the corneal cells of mammals and anurans (Krishnan et al., Reference Krishnan, Kathiresan, Raman, Rajini, Dhople, Aggrawal and Sharma2007; Jester, Reference Jester2008).
In the retina, crystallins with a 23-kDa molecular weight (corresponding to both αA and αB-crystallin) were described in frog (anuran) Müller cells (Simirskiĭ et al., Reference Simirskiĭ, Panova, Sologub and Aleĭnikova2003), and αA-crystallin was found in the photoreceptor cells of mice and rats (Deretic et al., Reference Deretic, Aebersold, Morrison and Papermaster1994; Maeda et al., Reference Maeda, Ohguro, Maeda, Nakagawa and Kuroki1999). In addition, αB-crystallin has been found in the soluble component of the porcine inter-photoreceptor matrix (Hauck et al., Reference Hauck, Schoeffmann, Deeg, Gloeckner, Swiatek-de Lange and Ueffing2005), in the pigment epithelium of patients with early and advanced age-related macular degeneration (AMD) (De et al., Reference De, Rabin, Salero, Lederman, Temple and Stern2007), in the retinal tissues of glaucoma patients (Funke et al., Reference Funke, Perumal, Beck, Gabel-Scheurich, Schmelter, Teister, Gerbig, Gramlich, Pfeiffer and Grus2016), and in experimental models of uveitis (Rao et al., Reference Rao, Saraswathy, Wu, Katselis, Wawrousek and Bhat2008) and of retinal degeneration (Cavusoglu et al., Reference Cavusoglu, Thierse, Mohand-Saïd, Chalmel, Poch, Van-Dorsselaer, Sahel and Léveillard2003). α-Crystallins are known to protect cells from stress-induced apoptosis, regulate cell growth, and enhance genomic stability (Andley, Reference Andley2009). The expression of both αA- and αB-crystallins increases after retinal insults, such as light toxicity and trauma (Sakaguchi et al., 2003; Vázquez-Chona et al., Reference Vázquez-Chona, Song and Geisert2004), and these proteins subsequently inhibit apoptosis by interacting with proapoptotic proteins of the Bcl-2 family (Mao et al., Reference Mao, Liu, Xiang and Li2004). Therefore, the accumulation of crystallins at the retinal pigment epithelium (RPE) interfaces (drusen, Bruch’s membrane, and choroid) in AMD patients has been described as a stress-related response of crystallins to the disease (Nakata et al., Reference Nakata, Crabb and Hollyfield2005). Similarly, in the lens α-crystallins trap aggregation-prone denatured proteins, helping to delay the development of age-related cataracts (Andley, Reference Andley2009).
Recently, there has been interest in the role of crystallins in neuroinflammation (Dulle & Fort, Reference Dulle and Fort2016) and in their presence in glia. Increased levels of expression of α-crystallins have been found in glial cells in several models of diseases related to neuroinflammation, such as Alzheimer’s disease (Renkawek et al., Reference Renkawek, Voorter, Bosman, van Workum and de Jong1994, Reference Renkawek, Stege and Bosman1999) and multiple sclerosis (van Noort et al., Reference van Noort, van Sechel, Bajramovic, el Ouagmiri, Polman, Lassmann and Ravid1995; Bajramović et al., Reference Bajramović, Lassmann and van Noort1997). This increase in expression has been correlated with the activation of astrocytes or astrogliosis, thus suggesting a role for α-crystallin in regulating the response of astrocytes to inflammatory signals. For example, in Alexander disease, mutated and upregulated glial fibrillary acidic protein filaments within astrocytes are colocalized with the upregulation of αB-crystallin (Koyama & Goldman, Reference Koyama and Goldman1999). The chaperone function of α-crystallins has also been described in nonglial cell types, but this α-crystallin function in inflammation may be specific for glial cells. In the retina, α-crystallins are increased in Müller glia in response to activation caused by diabetes (Fort et al., Reference Fort, Freeman, Losiewicz, Singh and Gardner2009); however, the chaperone properties of α-crystallin are disrupted by diabetes, contributing to the loss of neuroprotection (Losiewicz & Fort, Reference Losiewicz and Fort2011). Therefore, despite the increased expression of α-crystallin in different glial cell types, these proteins may have different roles in each cell type that need to be explored.
Crystallins and Transparency
Crystallins are important for cell transparency in both the cornea and the lens (Delaye & Tardieu, Reference Delaye and Tardieu1983). The major function of the cytoplasm in eye lens fiber cells is to form a highly refractive transparent medium that helps the lens to focus images on the retina. This highly refractive medium (n ranging from 1.37 to 1.44) is obtained by a high concentration of soluble proteins (Atchison & Smith, Reference Atchison and Smith1995). α-Crystallin is the major structural protein of the eye lens fiber cell cytoplasm that contributes to these physical properties of the lens. The extended sponge-like oligomeric structure of α-crystallin results in a highly hydrodynamic volume that reduces light scattering. Interestingly, and contrary to their role inside cells, α-crystallins in solution experience macromolecular changes that have been found to increase light scattering and solution opacity (Xia et al., Reference Xia, Wang, Tatarkova, Aerts and Clauwaert1996). Also, depletion of soluble α-crystallins leads to protein aggregation within the lens and eventually to cataracts (Datiles et al., Reference Datiles, Ansari, Suh, Vitale, Reed, Zigler and Ferris2008). In the lens, point mutations have been identified in the genes encoding α-, β-, and γ-crystallins that lead to the development of an hereditary form of human cataracts, either present at birth or developing at an early age (Graw, Reference Graw2009; Andley et al., Reference Andley, Tycksen, McGlasson-Naumann and Hamilton2018). Similarly, in the cornea, decreased expression of corneal crystallins in stromal keratocytes has been associated with increased in vitro and in vivo light scattering (Jester, Reference Jester2008).
There are many explanations for the effect of the crystallins on transparency and light-scattering. The traditional idea is that a tissue is transparent if there is no absorption or scattering. The principal reason for the nontransparency of organic tissues appears to be light scattering, because the majority of organic molecules do not absorb visible light (Delaye & Tardieu, Reference Delaye and Tardieu1983). Discontinuities in the refractive index inside the cytoplasm are the main reason for light scattering: as the light direction is altered when it passes through the boundary between regions of different refractive indices (e.g., cytoplasm and organelles), the tissue, though nonabsorbing, will be opaque (Johnsen, Reference Johnsen2001). This effect of refractive index discontinuities on light scattering explains the classical view of the effect of crystallins on transparency: they preserve short-range order in the organization of the cytoplasmic filaments and organelles, homogenizing mismatched refractive indices of individual cellular components (Xia et al., Reference Xia, Wang, Tatarkova, Aerts and Clauwaert1996; Horwitz et al., Reference Horwitz, Bova, Ding, Haley and Stewart1999). This ordering effect is due to the tertiary globular 3D-structure of α-crystallin and depends on the ratio of the subunits (Xia et al., Reference Xia, Wang, Tatarkova, Aerts and Clauwaert1996; Horwitz et al., Reference Horwitz, Bova, Ding, Haley and Stewart1999; Peschek et al., Reference Peschek, Braun, Franzmann, Georgalis, Haslbeck, Weinkauf and Buchner2009). It was shown also that α-crystallin maintains short-range order due to its interaction with specialized beaded intermediate filaments composed of filensin and phakinin proteins. In lens fiber cells, αA-crystallin decorates the filensin–phakinin filamentous core of the specialized beaded intermediate filaments found in these cells, as monomers spaced 7 nm apart or as dimers spaced 15 nm apart (the αA-crystallin motif), forming highly ordered 3D-matrices that enfold the massive quantities of crystallins expressed in fiber cells (Zampighi et al., Reference Zampighi, Zampighi and Lanzavecchia2011). This filamentous structure organizes the cytoplasmic proteins and organelles, thus clearing the path for light propagation without scattering (Benedek, Reference Benedek1971; Delaye & Tardieu, Reference Delaye and Tardieu1983). αA-crystallin, like other heat shock proteins, also reduces the molecular energy and stabilizes associated filaments, allowing them to withstand heat shocks (Horwitz, Reference Horwitz2009).
Another possible explanation of cell transparency is that specialized intermediate filaments decorated by αA-crystallin span the cell from one side to another and can transmit light energy all by themselves. It was calculated that, if composed of electro-conductive proteins, these structures could work as nanoscale light guides, using quantum confinement mechanisms (Zueva et al., Reference Zueva, Golubeva, Korneeva, Makarov, Khmelinskii and Inyushin2016; Makarov et al., Reference Makarov, Zueva, Golubeva, Korneeva, Khmelinskii and Inyushin2017). In this model, light scattering by organelles and protein particles is of no importance, because the light energy remains contained inside the filaments during light propagation.
Light Path in the Eye
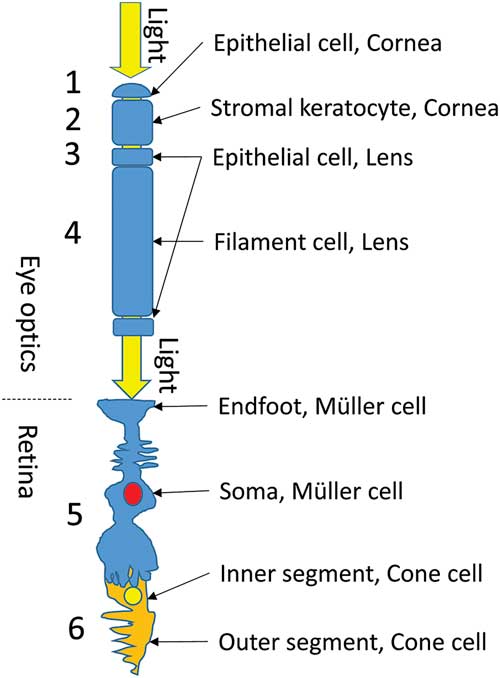
In the vertebrate eye, light passes through a sequence of specialized transparent cells before reaching the photoreceptors. First, it passes through the cornea, which consists of epithelial and stromal cells (Fig. 1, cells 1 and 2). The cornea has both beaded filaments (FitzGerald et al., Reference FitzGerald, Sun, Shibata and Hess2016) and α-crystallin (Krishnan et al., Reference Krishnan, Kathiresan, Raman, Rajini, Dhople, Aggrawal and Sharma2007; Jester, Reference Jester2008). The lens also has two types of cells, the small epithelial and long fiber cells (Fig. 1, cells 3 and 4), each of which also has beaded filaments (Quinlan et al., Reference Quinlan, Carte, Sandilands and Prescott1996; Clark et al., Reference Clark, Matsushima, David and Clark1999; Oka et al., Reference Oka, Kudo, Sugama, Asami and Takehana2008; Song et al., Reference Song, Landsbury, Dahm, Liu, Zhang and Quinlan2009) and α-crystallin in significant quantities (Delaye & Tardieu, Reference Delaye and Tardieu1983; Wistow & Piatigorsky, Reference Wistow and Piatigorsky1988; Horwitz et al., Reference Horwitz, Bova, Ding, Haley and Stewart1999; Zampighi et al., Reference Zampighi, Zampighi and Lanzavecchia2011; Andley et al., Reference Andley, Tycksen, McGlasson-Naumann and Hamilton2018). After the lens and the vitreous fluid, the light must pass through Müller cells (Fig. 1, cell 5) and then through the inner segment of the photoreceptor cell (Fig. 1, cell 6) to reach the outer segment (OS), where the photopigments are situated.

Figure 1 Light path through the vertebrate eye. Before reaching the photoreceptors, light must pass through a sequence of specialized transparent cells (1–6, see text).
Recently, high transparency was observed in retinal Müller cells, with the cell processes providing cellular light guidance through the inverted retina of vertebrates (Franze et al., Reference Franze, Grosche, Skatchkov, Schinkinger, Foja, Schild, Uckermann, Travis, Reichenbach and Guck2007; Reichenbach & Bringmann, Reference Reichenbach and Bringmann2013; Agte et al., Reference Agte, Junek, Matthias, Ulbricht, Erdmann, Wurm, Schild, Käs and Reichenbach2011). Cone photoreceptor inner segments are also well-known light guides (Enoch, Reference Enoch1963; Westheimer, Reference Westheimer2008). Do these cells also have α-crystallin-decorated intermediate filaments that limit light scattering? This is yet to be determined.
In the work presented in this article, we studied whether αA-crystallin, the main component of α-crystallin, is present in Müller cells and in the inner segments of photoreceptors in mammals (i.e., rat). We used immunohistochemistry, confocal imaging, and immunogold staining with transmission electron microscopy to visualize αA-crystallin protein in the retinal tissue.
Materials and Methods
Animals
Experiments were carried out with Institutional Animal Care and Use Committee approval and in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Retinae were obtained from the eyes of Sprague-Dawley rats (Rattus norvegicus). Rats (21 days old) were housed in a standard cage in a 12-h light–dark cycle room and had free access to food (standard rat chow) and water. The animals were sacrificed, and the eyes were rapidly enucleated and processed for immunohistochemistry.
Immunohistochemistry and Fluorescent Confocal Imaging of Retinal Tissues
Tissues were prepared for immunohistochemistry as described before (Zayas-Santiago et al., Reference Zayas-Santiago, Agte, Rivera, Benedikt, Ulbricht, Karl, Dávila, Savvinov, Kucheryavykh, Inyushin, Cubano, Pannicke, Veh, Francke, Verkhratsky, Eaton, Reichenbach and Skatchkov2014). Incised eyeballs were placed in a 4% paraformaldehyde (cat. #P6148; Sigma-Aldrich, St Louis, MO, USA) fixative solution in 0.1 M phosphate-buffered saline (PBS; 136.9 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) for 45 min. The eyes were punctured with a 25-Ga needle in the sclera and fixed for an additional 20 min with fresh fixative solution. Eyes were washed three times with 0.1 M PBS before extracting the retinae in a cold PBS solution using a stereoscopic microscope (Stereomaster, cat. #FW00-20-1613; Fisher Scientific, Waltham, MA, USA). Fixed retinae were embedded in 4% agarose (cat. #15510-019; Gibco-BRL, Gaithersburg, MD, USA) in 0.1 M PBS. A Leica VT1000 S Vibratome (Leica Biosystems Inc., Buffalo Grove, IL, USA) was used to create 70-μm rat retinal sections.
Retinal slices were moved to a 24-well plate and permeabilized for 20 min with 1% dimethyl sulfoxide (DMSO) (cat. #02196055; MP Biomedicals, Santa Ana, CA, USA), 0.3% Triton X-100 (cat. #T9284; Sigma-Aldrich) in 0.1 M PBS. The permeabilization solution was removed and replaced with a blocking solution containing 2% bovine serum albumin (BSA, cat. #A4503; Sigma-Aldrich) and, depending on the secondary antibody, either 5% normal horse serum (cat. #S-2000; Vector Laboratories, Burlingame, CA, USA) or 5% normal goat serum (cat. #S-1000; Vector Laboratories); 1% DMSO; and 0.3% Triton X-100 in 0.1 M PBS. The samples were then incubated for 1 h at room temperature while gently shaking. The blocking solution was replaced with a fresh solution containing primary antibodies and left shaking overnight at 4°C. We used a rabbit polyclonal antibody against αA-crystallin (1:200, cat. #ab5595; Abcam, Cambridge, UK), a mouse monoclonal antibody against αA-crystallin (1:200, cat. #sc-28306; Santa Cruz Biotechnology, Dallas, TX, USA), and a rabbit polyclonal antibody against the glial marker glutamine synthetase (GS, 1:200, cat. #sc-9067; Santa Cruz Biotechnology). Afterwards, the washing procedure was repeated, and the samples were incubated with secondary antibodies diluted in the permeabilization solution. Anti-rabbit FITC (1:200, cat. #FI-1200; Vector Laboratories) and anti-mouse Texas Red (1:200, cat. #FI-2100; Vector Laboratories) were used. Samples were washed again with PBS and then mounted on slides. Hardset Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (cat. #H-1500; Vector Laboratories) was used to seal the slides and to stain the nuclei. Slides were examined with an Olympus BX60 microscope (Olympus, Tokyo, Japan) outfitted with the Olympus FV1000 confocal laser-scanning system. Immunolocalization of αA-crystallin in retinal whole mounts followed the same procedure but was modified by having a 48-h primary antibody incubation, followed by a 24-h incubation with secondary antibodies.
Immuno-Electron Microscopy
Retinal tissues from rat were prepared for immuno-electron microscopy according to Schikorski (Reference Schikorski2010). Eyes were placed in a fixative solution containing 4% paraformaldehyde and 0.1% glutaraldehyde (cat. #G7651; Sigma-Aldrich) in 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES)-buffered saline (HBS; 154 mM NaCl, 0.2 mM CaCl2, 20 mM HEPES, pH 7.4) for 1 h. Eyes were punctured with a 25-Ga needle in the sclera and fixed for an additional 30 min with fresh fixative solution. Eyes were washed three times with HBS before extracting the retinae in a cold HBS solution using a stereoscopic microscope. Fixed retinae were embedded in 4% agarose in HBS. A Leica VT1000 S Vibratome was used to create 125-μm retinal sections. Samples were moved to a 24-well plate and permeabilized and blocked with a solution containing 10% BSA, 0.03% Triton X-100 in HBS, pH 7.4, for 30 min while shaking. The permeabilization solution was removed, the primary antibody solution was added (αA-crystallin antibody, diluted 1:300 with 1% BSA, 0.03% Triton X-100 in HBS), and the samples were left shaking overnight at 4°C. The primary antibody solution was removed, and the samples were washed with 0.05% BSA in HBS for 5 min three times. After washing, the secondary antibody solution (anti-rabbit IgG labeled with 5-nm colloidal gold, 1:100, cat. #G7277; Sigma-Aldrich) was added and left to shake at room temperature for 3 h. Next, the washing procedure was repeated before moving samples with a brush to a glass vial containing Karnovsky’s fixative (4% paraformaldehyde, 5% glutaraldehyde, 0.2 mM CaCl2, 90 mM sodium cacodylate, pH 7.4), where they were left to be fixed for 7 days at 4°C.
Samples were moved again to a 24-well plate and washed three times for 10 min with cacodylate (CaCo) buffer (90 mM sodium cacodylate, 0.2 mM CaCl2, pH 7.4). Next, the slices were postfixed in 1% OsO4 with 1.5% K4[Fe(CN)6] for 30 min in the same buffer, then washed twice for 10 min with pure CaCo buffer, postfixed a second time with 1% OsO4 in CaCo buffer for 30 min, washed once in CaCo buffer for 10 min, and then washed three times for 5 min in distilled water. Uranyl acetate solution was not used, in order to avoid contaminating the gold particles with uranium. Dehydration was carried out through an ascending series of ethanol–water solutions (50, 70, 90, and 100% ethanol) for 10 min each, and then incubation in 100% ethanol was repeated three times for 10 min to ensure complete dehydration.
The 100% ethanol was replaced with a 1:1 mixture of 100% ethanol and 1:1 Epon/Spurr EMbed 812 EMS embedding medium (Electron Microscopy Sciences, Hatfield, PA, USA) and left to infiltrate overnight. Next, this ethanol:Epon/Spurr mixture was replaced with pure 1:1 Epon/Spurr medium and left to infiltrate for 2 h, replaced again with a fresh solution, left for 2 h before replacing with fresh solution once more, and left to infiltrate overnight. Finally, the tissues were oriented and polymerized at 60°C for 48 h. Ultrathin (60-nm) sections were made using an LKB Ultratome (LKB-Produkter, Bromma, Sweden) and examined using a JEM-100B electron microscope (JEOL Ltd., Tokyo, Japan).
Results
Immunohistochemistry
Expression of αA-Crystallin in the Rat Retina
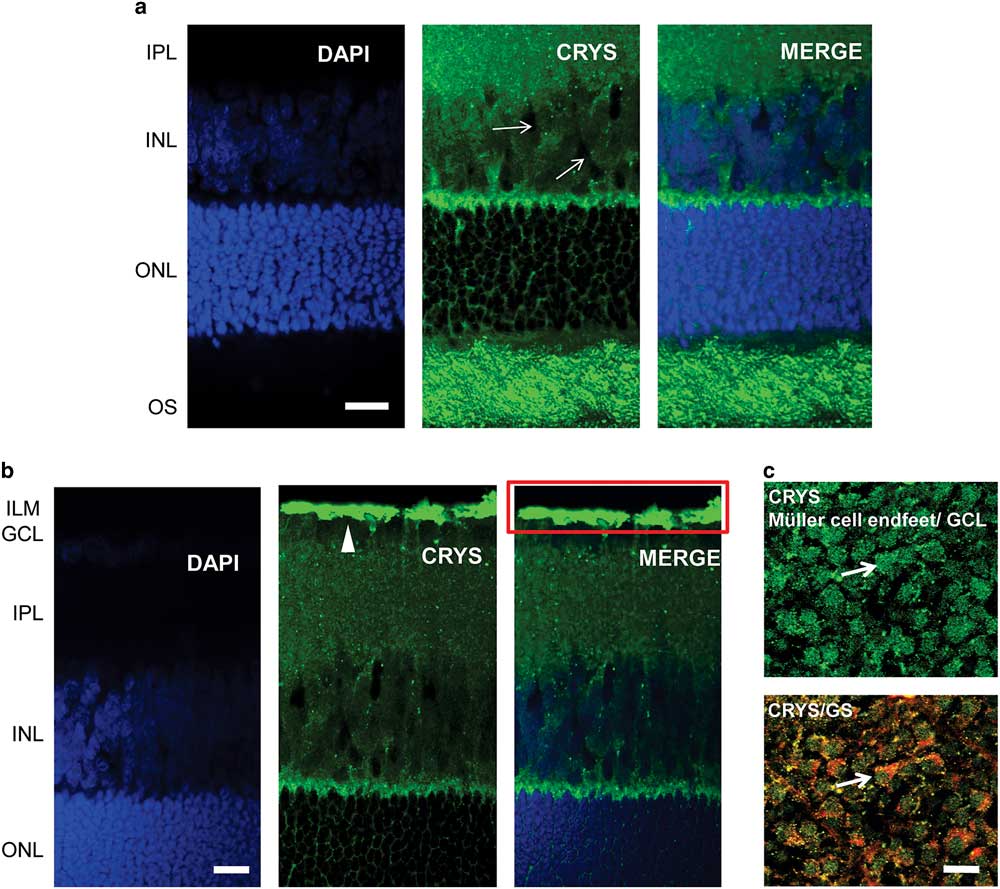
We used immunohistochemistry to observe the localization of αA-crystallin in the rat retina. We show the results for the localization of αA-crystallin from the photoreceptor OS area to the inner plexiform layer in Figure 2a and from the outer nuclear layer (ONL) to the inner limiting membrane (ILM) in Figure 2b. αA-Crystallin was observed to be highly expressed in the OS area of photoreceptors and in the contact area between Müller cells and the synapses of photoreceptors [between the inner nuclear layer (INL) and ONL, Fig. 2a]. From the images we cannot conclude whether αA-crystallin is found within the inner or the OSs or in the area surrounding them. This contact area, between the INL and ONL, is where the Müller cell soma area connects with its distal processes.
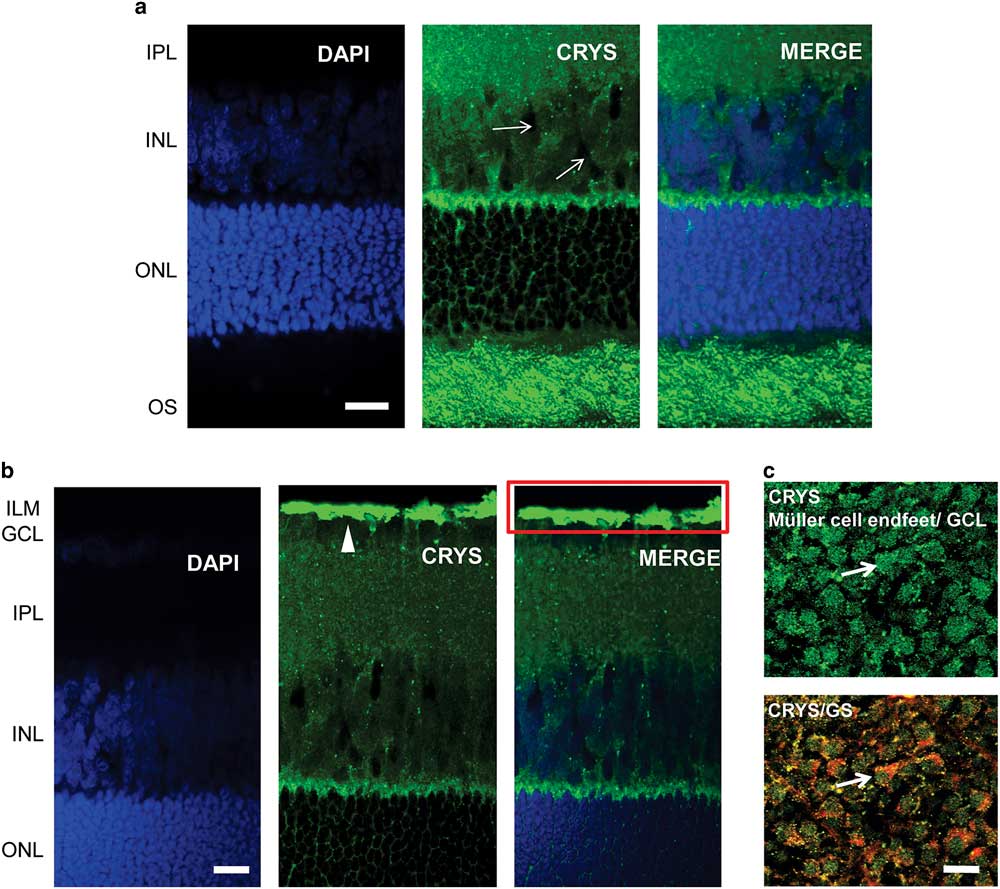
Figure 2 Immunolocalization of αA-crystallin in the rat retina. a: αA-crystallin (CRYS, green) is expressed in the inner nuclear layer (INL) within the Müller cell compartments (white arrows) surrounding the nuclei of neurons (DAPI nuclear staining, blue) and in the contact area between Müller cells and the synapses of photoreceptors (between the INL and ONL). Expression was also localized to the outer segment area of photoreceptors. b: αA-crystallin was highly expressed in the inner limiting area above the ganglion nuclear layer (white arrowhead). The red rectangle corresponds to the ganglion cell layer (GCL) and the Müller endfeet area, which is observed in (c) from the top. c: Upper image: αA-crystallin observed from the top of a whole-retinal tissue. αA-crystallin is confined to the endfeet of Müller cells in the GCL (white arrow). Lower image: merged image of αA-crystallin (CRYS, green) and the glial marker glutamine synthetase (GS, red). GS is observed surrounding the borders of the endfeet (white arrow). IPL, inner plexiform layer; ILM, inner limiting membrane; ONL, outer nuclear layer; OS, outer segments of photoreceptors. Scale bar is 20 μm.
αA-Crystallin was also observed within the INL in the Müller cell compartments surrounding the neuronal nuclei. It is in this area that the soma of Müller cells is located in between the other neuronal nuclei. Also, it is evident that αA-crystallin is located within the distal processes of Müller cells in the ONL, branching and surrounding the cell nuclei of photoreceptors. High expression of αA-crystallin was also found in the ILM above the ganglion cell layer (Fig. 2b). The ILM area is where the endfeet of Müller cells make a border between the retina and vitreal humor and where the Müller cell endfeet are mostly concentrated.
We evaluated the expression of αA-crystallin in whole rat retinal tissue and observed it from the vitreal surface. We found that αA-crystallin was concentrated within islands that correspond to the endfeet of Müller cells (Fig. 2c). The lower image in Figure 2c shows the merged expression of antibodies for both αA-crystallin and the glial marker GS (red). GS is observed surrounding the borders of the endfeet (white arrow). Areas of colocalization that appear in yellow and orange are also visible within the endfeet.
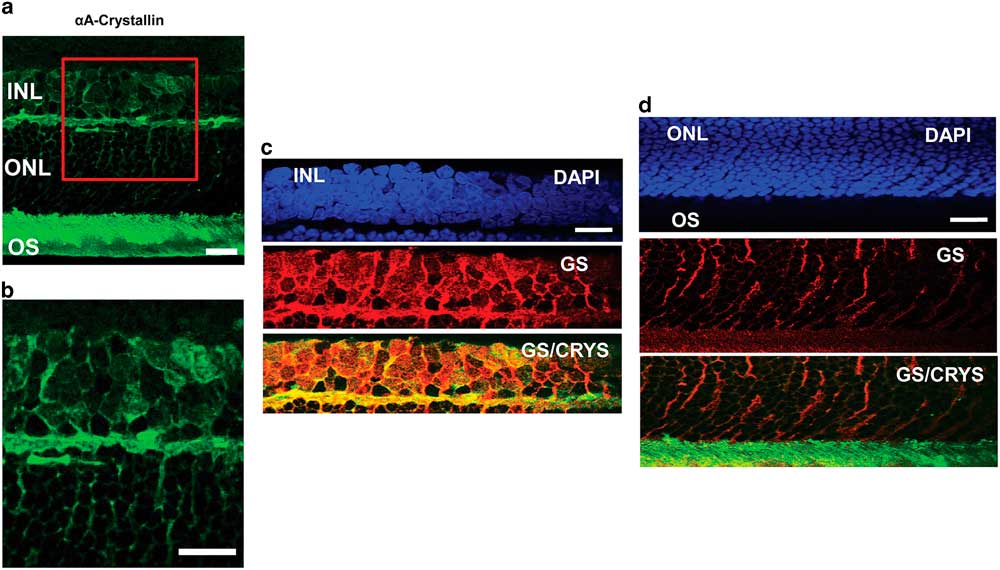
To confirm the localization of αA-crystallin within the Müller glial cell compartments, we studied its colocalization with the glial marker GS in other retinal layers as well (Fig. 3). In an enlarged image of the INL and ONL (Figs. 3a, 3b) one can observe αA-crystallin around and within some of the nuclei present in the INL, and it clearly enwraps the nuclei in the ONL. We show the expression of αA-crystallin and GS in the INL (Fig. 3c), the ONL (Fig. 3d), and the photoreceptor inner/OS region (Fig. 3d). A strong colocalization was observed with GS in the INL and the synaptic region between the INL and the ONL. The glial marker GS strongly labels Müller cell soma present within the INL, and it coincides with αA-crystallin expression. Other neuronal nuclei in this area appear dark and exclude both markers. In the ONL, GS expression corresponds to Müller cell distal processes that surround neuronal nuclei and coincides with the pattern of expression of αA-crystallin in this region.
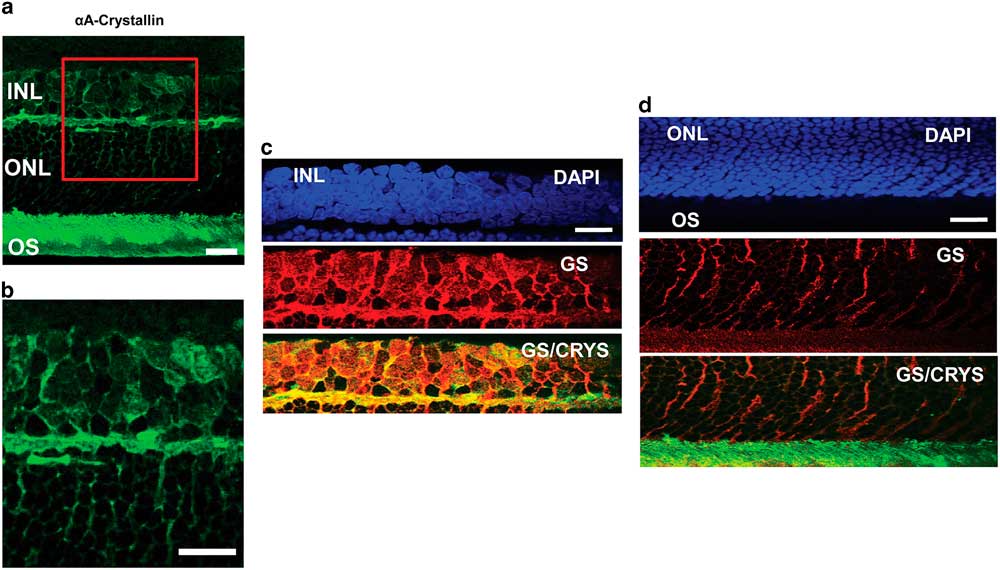
Figure 3 Immunolocalization of αA-crystallin and glutamine synthetase (GS) within the Müller glial cell compartments. a: The expression of αA-crystallin (green) in the rat retina inner nuclear layer (INL), the outer nuclear layer (ONL), and the photoreceptor inner/outer segment (OS) region. b: Enlarged view of the area in the red square in (a). c: INL: expression of nuclear staining (DAPI, blue), glial marker GS (red), and merging of αA-crystallin (green) and GS (red). d: ONL and the photoreceptor inner/OS region: expression of nuclear staining (DAPI, blue), glial marker GS (red), and merging of αA-crystallin (green) and GS (red). Scale bar is 15 μm.
Electron Microscopy: Localization of αA-Crystallin in the Rat Retina with Immunogold
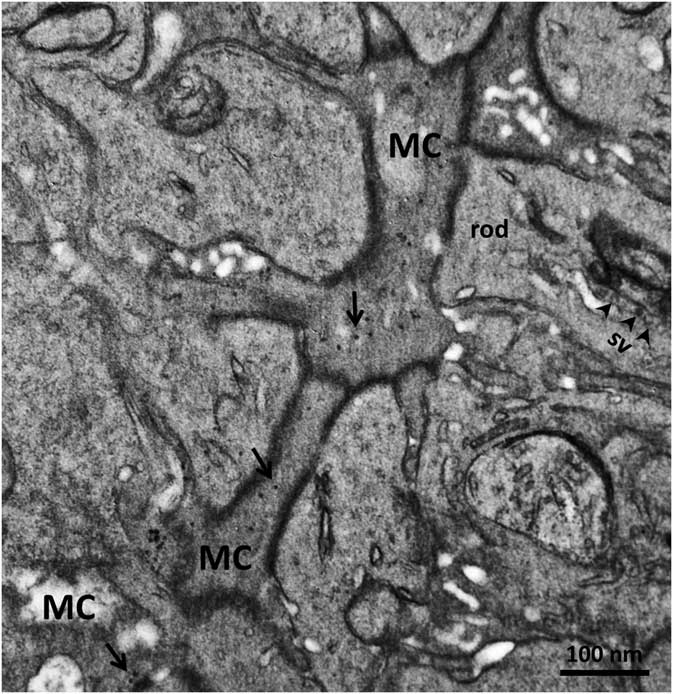
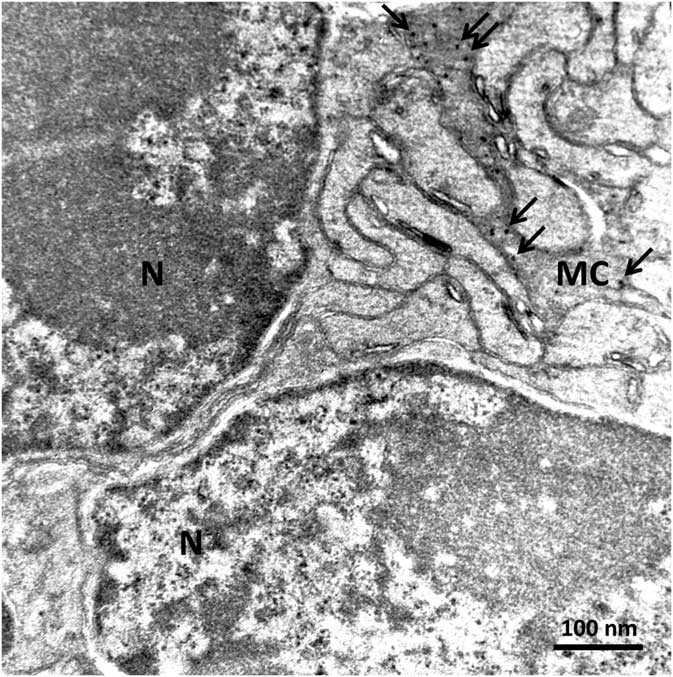
In order to confirm the presence of αA-crystallin inside Müller cells, we used electron microscopy on rat retina stained with antibodies against this protein and poststained with secondary antibodies conjugated with 5-nm colloidal gold particles (immunogold, see Methods). The ultrathin sections were made at all retinal levels to identify the different parts of Müller cells that span the whole retina. The cells extend from the inner limiting membrane, where the light enters the retina and passes through the entire retina to the OS of the photoreceptor cells (Fig. 1). It is well known that the cytoplasm of Müller cells in electron micrographs are more osmiophilic and look darker than the neuronal cytoplasm (Jeon et al., Reference Jeon, Strettoi and Masland1998; Zueva et al., Reference Zueva, Golubeva, Korneeva, Makarov, Khmelinskii and Inyushin2016), and this darker appearance allows easy recognition of Müller cell processes. Using this and other morphological criteria for recognizing Müller cells, we found that immunogold particles were concentrated in the cytoplasm of Müller cells and also within the nuclei of some neurons (Figs. 4, 5). As an example, we present electron micrographs of Müller cells from different parts of the retina. In Figure 4 we display a rat retina transversal section close to the level of the rod photoreceptor synaptic terminals. Müller cell processes look darker and contain immunogold particles (marked by arrows), while nearby processes of different neuronal cells contain no gold particles. Figure 5 presents the transversal section of the rat retina at the inner nuclear level. Similarly, only the processes of the Müller cells, which look darker, contain gold particles of standard size (5 nm, arrows). The nuclei of nearby neurons (bipolar cells) contain black particles of heavy metals, but as the majority of these particles are of different sizes, it is impossible to completely exclude the possibility that some gold particles are also present. Note that we did not use uranyl acetate staining, which may yield artefactual particles that look similar to gold particles in immunogold staining (Schikorski, Reference Schikorski2010).
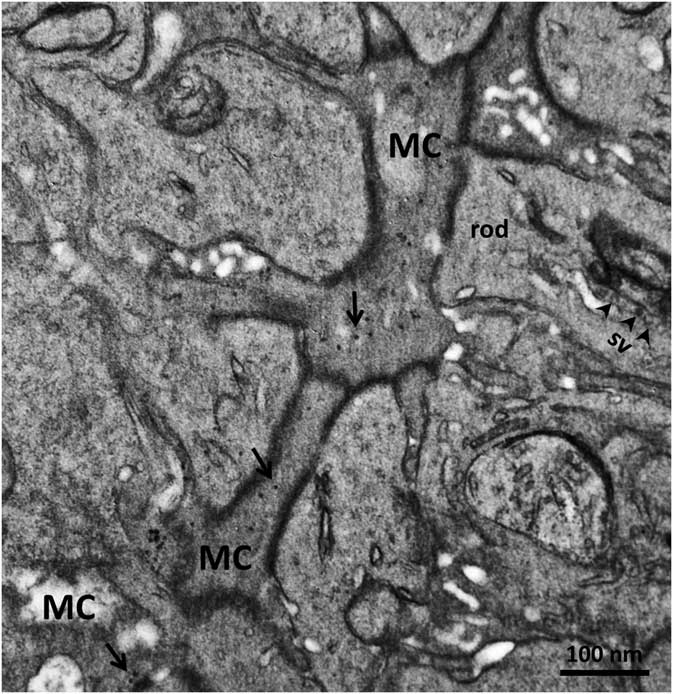
Figure 4 Electron micrograph of a transversal section of rat retina at the rod photoreceptor synaptic terminal level. Synaptic vesicles (sv) are marked with arrowheads. The cytoplasm of Müller cells (MC) are more osmiophilic than the cytoplasm of nearby cells and look darker. Standard 5-nm particles of immunogold (long arrows) are found only in these darker processes. Scale bar is 100nm.
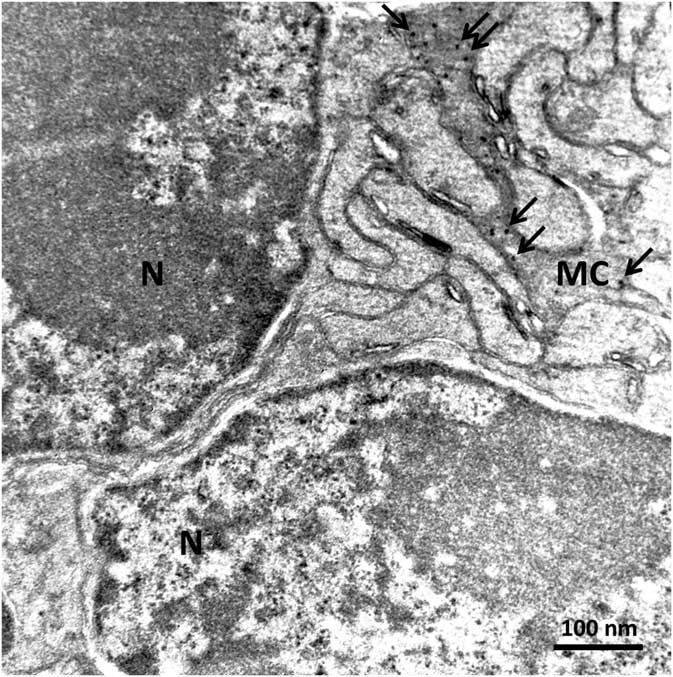
Figure 5 Electron micrograph of a rat retina transversal section at the inner nuclear layer. The dark osmiophilic Müller cell (MC) process is located between the nuclei (N) of two bipolar cells. Only the Müller cell processes contain standard 5-nm particles of immunogold (arrows). Scale bar is 100 nm.
Discussion
Using immunocytochemistry, fluorescence confocal imaging, and immuno-electron microscopy we examined the distribution of αA-crystallin, the main component of the α-crystallin complex, in the rat retina (a convenient mammalian model). αA-crystallin immunofluorescence was observed in the retina using anti-αA-crystallin antibodies. We observed that the cytoplasm of retinal Müller cells, as well as their endfeet, were clearly marked with αA-crystallin immunofluorescence (Figs. 2, 3). A transversal cut through the retina and the view from the top of a retinal whole mount specifically exposed αA-crystallin in the Müller cell endfeet (Fig. 2c), which was colocalized with the glial marker GS. αA-crystallin immunofluorescence is clearly observed radially surrounding retinal neurons at the level of the ONL and INL (Figs. 2a, 2b; 3a–3d). This is probably because columnar organization is a characteristic feature of vertebrate retinae, and Müller cell processes are involved in this organization (Reichenbach & Robinson, Reference Reichenbach and Robinson1995). It was determined that Müller glia mechanically stabilize different cells within columns with their processes (Willbold et al., Reference Willbold, Reinicke, Lance-Jones, Lagenaur, Lemmon and Layer1995), and we have now found that these processes become visible with αA-crystallin immunofluorescence. The localization of αA-crystallin within Müller glial cells was confirmed by evaluating its colocalization with the GS marker (Fig. 3). A strong colocalization was observed in the INL (Fig. 3c), which corresponded to the Müller cell soma region, and in the synaptic region in between the INL and the ONL. In the INL, the expression of αA-crystallin matched the expression of GS found in the Müller cell stalks (Fig. 3d).
In order to confidently confirm the presence of αA-crystallin in small Müller cell processes, we used immuno-electron microscopy. Small particles of colloidal gold of a standard size (5 nm) can be recognized exclusively in osmiophilic Müller cell processes in different regions of the rat retina (Figs. 4, 5). Given that Müller glial cells were recently described as living light guides (Franze et al., Reference Franze, Grosche, Skatchkov, Schinkinger, Foja, Schild, Uckermann, Travis, Reichenbach and Guck2007; Reichenbach & Bringmann, Reference Reichenbach and Bringmann2013; Agte et al., Reference Agte, Junek, Matthias, Ulbricht, Erdmann, Wurm, Schild, Käs and Reichenbach2011), we suggest that αA-crystallins, as with other highly transparent cells, could enable a high refractive index within Müller cells, which helps to trap and guide light in retinal tissue. αA-crystallin immunofluorescence clearly marks the inner and OSs of photoreceptors (Figs. 2a; 3a, 3d), which we suggest may be connected to the light pathway, as photoreceptors are also well-known light guides (Enoch, Reference Enoch1963; Westheimer, Reference Westheimer2008). αA-crystallin may be contained in the matrix, as another type of crystallin (αB-crystallin) was found previously in the soluble component of the porcine inter-photoreceptor matrix (Hauck et al., Reference Hauck, Schoeffmann, Deeg, Gloeckner, Swiatek-de Lange and Ueffing2005).
It has also been recognized that, as a member of the heat shock protein family, αA-crystallin can enhance the survival of a retinal cell under challenging conditions. This was shown by artificial transfections leading to expression of αA-crystallin in retinal ganglion cells (van den IJssel et al., Reference van den IJssel, Overkamp, Knauf, Gaestel and de Jong1994; Caprioli et al., Reference Caprioli, Munemasa, Kwong and Piri2009) and by exogenous supplementation of α-crystallin that was taken up by the lens, thereby delaying the onset of cataracts (Lin & Lavail, 2010). Moreover, these proteins are dramatically upregulated in a range of retinal diseases, including diabetic retinopathy, AMD, uveitis, trauma, and ischemia, although the regional distribution of this overexpression is not well characterized (reviewed in Fort & Lampi, Reference Fort and Lampi2011). Interestingly, in the inflammatory autoimmune disease of uveitis, in which Müller cells become disrupted, the retina appears “milky” and opaque (Eberhardt et al., Reference Eberhardt, Amann, Feuchtinger, Hauck and Deeg2011). This is similar to the differences in opacity observed in solutions of α-crystallin with altered macromolecular structure (Xia et al., Reference Xia, Wang, Tatarkova, Aerts and Clauwaert1996), suggesting that crystallins are important for maintaining transparency in a healthy retina and that a uveitis patient may benefit from exogenous supplementation of α-crystallin, to delay the onset of protein aggregation (Mueller et al., Reference Mueller, Fogueri, Pedler, Montana, Petrash and Ammar2015).
This finding is no surprise, as αA-crystallin may have a powerful chaperone function similar to αB-crystallin. But unlike αB-crystallin, αA-crystallin is polydisperse, and, in addition to polymeric complexes of 24 subunits, smaller oligomers and large clusters consisting of individual oligomers may also be present (Peschek et al., Reference Peschek, Braun, Franzmann, Georgalis, Haslbeck, Weinkauf and Buchner2009). αA-crystallin also decorates the filensin–phakinin filamentous core of specialized beaded intermediate filaments, as monomers spaced 7 nm apart or as dimers spaced 15 nm apart (the αA-crystallin motif), forming highly ordered 3D-matrices that enfold massive amounts of αA-crystallin (Zampighi et al., Reference Zampighi, Zampighi and Lanzavecchia2011). The structural differences described may be the basis for the optical functionality attributed to this protein. Given that Müller glial cells and cones were recently described as living light guides, we suggest that αA-crystallin, as in other highly transparent cells, allows Müller cells and cones to minimize intraretinal scattering during retinal light transmission. Still, since crystallins are known to be present in other retinal cells as well, such as in the RPE (De et al., Reference De, Rabin, Salero, Lederman, Temple and Stern2007), the specific role of these proteins in transparency and light scattering in the retina still needs to be investigated.
Conclusions
Using immunocytochemistry, fluorescent confocal imaging, and immuno-electron microscopy, we found that αA-crystallins are present in the cytoplasm of rat retinal Müller cells as well as in the photoreceptor cells. We suggest that an important novel role of αA-crystallins in these retinal cells is the reduction of optical light scattering.
Acknowledgments
The authors would like to thank Alexandra Matos Santiago and Ismael Pérez for their technical contribution to this work. This work was financially supported by the US Department of Education (USDE-Title-V-PO31S130068 to A.Z-S.), the NIH NIGMS (SC2GM111149 for M.Y.I.), and an RSF grant (16-14-10159 to L.V.Z.).