Article contents
ITGB1 Enhances the Proliferation, Survival, and Motility in Gastric Cancer Cells
Published online by Cambridge University Press: 29 July 2021
Abstract
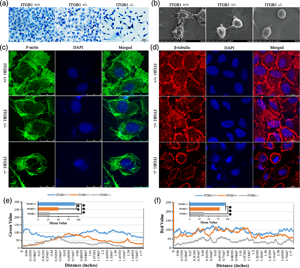
ITGB1 (Integrin β1, CD29) is a member of the integrin family and has a role as a major adhesion receptor. Gastric cancer (GC) is an important cause of mortality worldwide, especially in China. As a potential cancer enhancer, the role ITGB1 plays in GC progression remains unclear. In the current study, our assay on the databases of tumoassociated gene expression and interaction found that the high expression of ITGB1 was closely correlated with the poor prognosis of GC patients. To explore the roles, ITGB1 plays in GC progression, and an ITGB1-deleted cell line (ITGB1−/−SGC7901) was generated using the CRISPR/Cas9 method. The tumor malignancy-associated cell behaviors and microstructures were detected, imaged, and analyzed using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), wound healing, transwell, scanning electron microscopy, laser scanning confocal microscopy, and others. The results indicated that ITGB1 deletion decreased the GC cell proliferation and motility, and inhibited motility-relevant microstructures, such as pseudopodia and filopodia, markedly in ITGB1-deleted SGC7901 cells. The analysis of STRING database and western blots indicated that ITGB1 contributes to the malignancy of GC mediated by Src-mediated FAK/PI3K/Akt signaling pathways. Taken together, the results showed that ITGB1 may be a potential targeting marker for GC diagnosis and therapy in the future.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 10
- Cited by



