No CrossRef data available.
Published online by Cambridge University Press: 23 May 2022
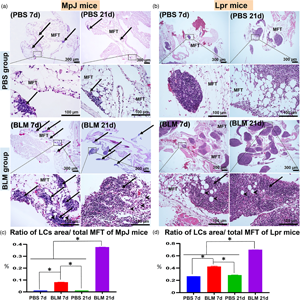
The purpose of this study is to elucidate the impact of bleomycin on the degree of lung injury and development of mediastinal fat-associated lymphoid clusters (MFALCs) in the lymphoproliferative mouse model (MRL/MpJ-Faslpr/lpr “Lpr”) and its control strain (MRL/MpJ “MpJ”). We analyzed immune cells, the degree of proliferation, lymphatic vessels (LVs), and high endothelial venules (HEVs) in lungs and MFALCs in Lpr and MpJ mice on the 7th and 21st days following intranasal instillation of either bleomycin (BLM group) or PBS (PBS group). The BLM group showed a significant increase in the size of MFALCs, lung injury score, and positive area ratios of LVs, HEVs, and immune cells (especially macrophages, B- and T-lymphocytes) on both days 7 and 21. Interestingly, the lungs in the BLM group on day 21 showed higher collagen deposition and cellular infiltration in MpJ and Lpr, respectively. Moreover, significant positive correlations were observed between the size of MFALCs and lung injury. In conclusion, BLM could exert lung fibrosis or lymphoproliferative infiltration in chronic stages in MpJ and Lpr, respectively, and this varied effect could be due to the variations in the degree of immune cell proliferation and the development of LVs and HEVs among the studied strains.