Published online by Cambridge University Press: 27 October 2020
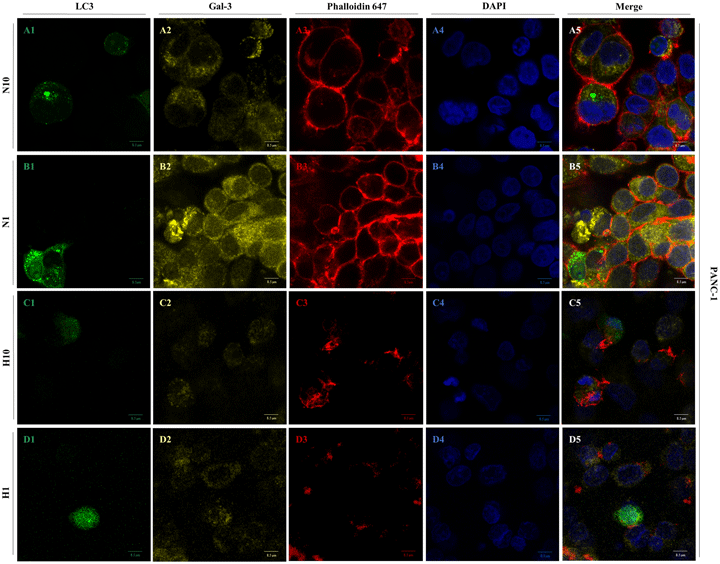
Hypoxia and nutrient deprivation are responsible for inducing malignant behavior in neoplastic cells. In these conditions, metabolic stress leads the cells to enhance their autophagic flux and to activate key molecules for homeostasis maintenance. Galectin-3 (Gal-3) is upregulated in pancreatic cancer and it is activated under the hypoxic atmosphere. We aimed to analyze the most effective autophagic-inducing conditions in pancreatic ductal adenocarcinoma cells and the effect exerted under these conditions in association with hypoxia on the Gal-3 expression. Gal-3 and the microtubule-associated protein light chain 3 beta (LC3) were accessed through western blot and immunofluorescence. Degradative vacuole quantification was analyzed by transmission electronic microscopy, and inhibition of Gal-3 was performed using siRNA. According to the analyses, the most effective conditions in the inducement of autophagy for PANC-1 and MIA PaCa-2 cells were nutritional deprivation and complete amino acid/glucose deprivation, respectively. PANC-1 cells presented higher Gal-3 when they were submitted to 24 h of nutritional deprivation alone and simultaneously nutritional and oxygen deprivation. Inhibition of Gal-3 causes a decrease of LC3 levels in all experimental conditions. These results confirm that Gal-3 is modulated by microenvironment factors and the possibility of Gal-3 participating in an adaptive response from PDAC cells to extreme conditions.