No CrossRef data available.
Article contents
The Electronic Structure and Bonding of Copper Oxides by CBED and EELS
Published online by Cambridge University Press: 02 July 2020
Extract
Cu2O and CuO are of great interest after the discovery of the high Tc superconductors. The superconductivity is believed to be dependent on charge states in CuO2 layers whose structure is similar to that of CuO. These transition-metal compounds are also important because of the different copper coordination numbers and different copper-oxygen distances, therefore these reflect different electron density distributions and bonding mechanisms. The electronic structure of cuprite has been studied extensively both theoretically and experimentally because of its symmetrical crystallographic structure (space group 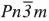 ) while that of CuO (space group C2/c) which has a monoclinic structure has been studied only recently. Previous experimental and theoretical results show that the valence electrons of copper in cuprite form
) while that of CuO (space group C2/c) which has a monoclinic structure has been studied only recently. Previous experimental and theoretical results show that the valence electrons of copper in cuprite form 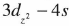 hybrid states which is proposed by Orgel instead of spherical d10 state as in ionic copper.
hybrid states which is proposed by Orgel instead of spherical d10 state as in ionic copper.
- Type
- Microscopy of Semiconducting and Superconducting Materials
- Information
- Copyright
- Copyright © Microscopy Society of America


