Article contents
Differential Distribution of Flavonoids and Phenolic Acids in Leaves of Kalanchoe delagoensis Ecklon & Zeyher (Crassulaceae)
Published online by Cambridge University Press: 19 August 2020
Abstract
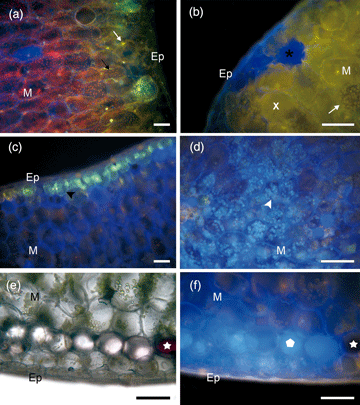
Kalanchoe delagoensis is adapted to intense solar irradiation, drought, and heat, partially due to the presence of phenols, important photo-protective compounds and antioxidants. This study aimed to evaluate the distribution of flavonoids and phenolic acid derivatives throughout the erect-tubular leaves of K. delagoensis. Specimens grown under sunny conditions were used for histochemical and high-performance liquid chromatography coupled with diode array detection (liquid HPLC-DAD) analysis. The NP (2-aminoethyl diphenylborinate) test suggested the presence of phenolic acids throughout the leaf blade below the epidermis and in chloroplasts, mainly in the leaf base. Flavonoids were detected specifically in chloroplasts, on the adaxial side of the middle third and at the leaf apex, near the meristematic cells. There was a tendency of flavonoid accumulation from the middle third to the apex, especially surrounding the gem, while phenolic acids were observed mainly in the base. This can be explained by the more exposed leaf apex and to the presence of apical buds (high production and regulation sites of ROS). The HPLC-DAD analysis showed different classes of flavonoids and phenolic acid derivatives in the leaf extracts, agreeing with the NP test results. This is the first time that the substitution of phenolic acids by flavonoids from the leaf base to the apex has been described.
Keywords
- Type
- Micrographia
- Information
- Copyright
- Copyright © Microscopy Society of America 2020
References
- 7
- Cited by



