Published online by Cambridge University Press: 15 October 2021
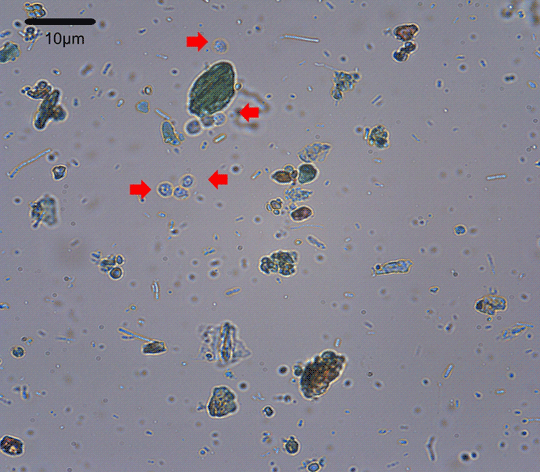
Interpretation errors may still represent a limiting factor for diagnosing Cryptosporidium spp. oocysts with the conventional staining techniques. Humans and machines can interact to solve this problem. We developed a new temporary staining protocol associated with a computer program for the diagnosis of Cryptosporidium spp. oocysts in fecal samples. We established 62 different temporary staining conditions by studying 20 experimental protocols. Cryptosporidium spp. oocysts were concentrated using the Three Fecal Test (TF-Test®) technique and confirmed by the Kinyoun method. Next, we built a bank with 299 images containing oocysts. We used segmentation in superpixels to cluster the patches in the images; then, we filtered the objects based on their typical size. Finally, we applied a convolutional neural network as a classifier. The trichrome modified by Melvin and Brooke, at a concentration use of 25%, was the most efficient dye for use in the computerized diagnosis. The algorithms of this new program showed a positive predictive value of 81.3 and 94.1% sensitivity for the detection of Cryptosporidium spp. oocysts. With the combination of the chosen staining protocol and the precision of the computational algorithm, we improved the Ova and Parasite exam (O&P) by contributing in advance toward the automated diagnosis.