Article contents
Quantification of Nanoparticles in Dispersions Using Transmission Electron Microscopy
Published online by Cambridge University Press: 11 May 2021
Abstract
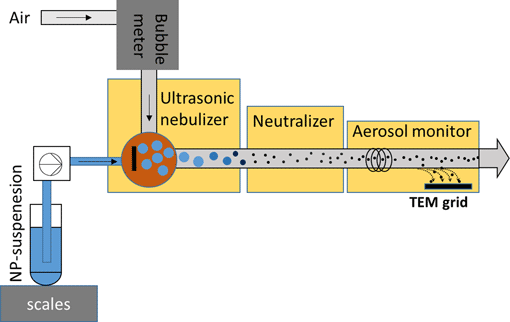
The quantification of the particle size and the number concentration (PNC) of nanoparticles (NPs) is key for the characterization of nanomaterials. Transmission electron microscopy (TEM) is often considered as the gold standard for assessing the size of NPs; however, the TEM sample preparation suitable for estimating the PNC based on deposited NPs is challenging. Here, we use an ultrasonic nebulizer (USN) to transfer NPs from aqueous suspensions into dried aerosols which are deposited on TEM grids in an electrostatic precipitator of an aerosol monitor. The deposition efficiency of the electrostatic precipitator was ≈2%, and the transport efficiency of the USN was ≈7%. Experiments using SiO2 NPs (50–200 nm) confirmed an even deposition of the nebulized particles in the center of the TEM grids. PNCs of the SiO2 NPs derived from TEM images underestimated the expected PNCs of the suspensions by a factor of up to three, most likely resulting from droplet coagulation and NP aggregation in the USN. Nevertheless, single particles still dominated the PNC. Our approach results in reproducible and even deposition of particles on TEM grids suitable for morphological analysis and allows an estimation of the PNC in the suspensions based on the number of particles detected by TEM.
- Type
- Software and Instrumentation
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 2
- Cited by



