Article contents
Evaluation of Corrosion of Refractory Materials Using Electron Microscopy
Published online by Cambridge University Press: 23 July 2021
Abstract
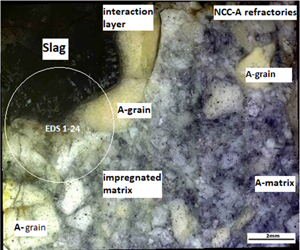
The interaction of NCC-A (no cement content, with tabular corundum) refractory castable with corrosive refining steel slag CaO–Al2O3–SiO2–MgO–(FeO, MnO) was studied with static crucible corrosion tests. The corrosion process was evaluated using chemical and phase analyses, and the macro- and microstructures on the corrosion boundary of slag–refractory materials were investigated. We describe the corrosion progress and explain the NCC-A corrosion mechanism by oxide metallurgical slags. The alumina refractory material surface was wetted with molten oxide slag (contact angle θ1390°C from 10° to 20°). The slag penetrated through dissolution of the matrix (fine alumina cement), and the Ca–Si–Al slag, enriched with alumina, attacked the corundum grog. Marked changes in the chemical composition of the slag had an impact on the primary crystallization temperature. As the concentration of Al–O complexes increased in the slag, the binding parameters in Si–O complexes changed and complex tetrahedral units (Al–O–Si) and (Al–O–Mg) were created. Analyses of the corrosion interface grog show that calcium ions diffuse through structural defects in the corundum but Mg2+ diffusion was not observed. A sharp interface is observed between the slag and the corroded corundum layer. Differences in the chemical composition of the slag in the corrosion zone affected the convention stream, and formed the concave corrosion profile of the crucible.
- Type
- The XVIIth International Conference on Electron Microscopy (EM2020)
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 1
- Cited by


