Published online by Cambridge University Press: 06 July 2021
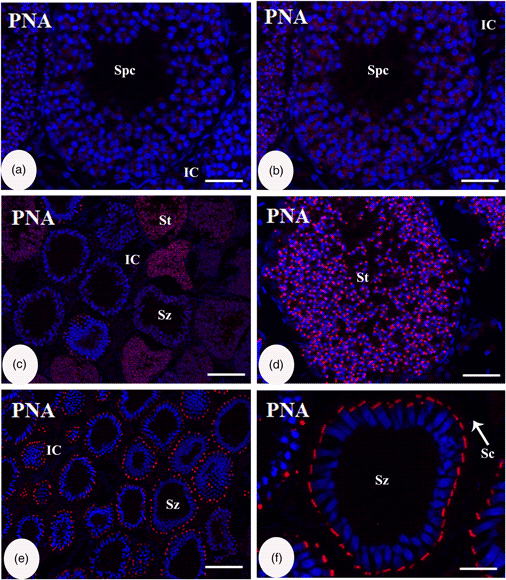
The testis of bamboo shark is characterized by diametric development leading to zonation architecture. Here, we investigated the staining pattern of 12 lectins in 6 groups of differential binding specificities within the germ, somatic, and interstitial cells of each zone. The neutral mucopolysaccharides appeared in the interstitial tissue in all the zones and became more significant in the spermatozoal–Sertoli cell junction. The cellular localization of the lectins varies in testicular zones and cell types. There was a gradual increase in glycosylation toward the degenerative zone. The increased intensity of most lectins in the interstitial cells indicates the association of glycoconjugates in their androgen-secreting activity. Statistical analyses showed a significant correlation between the groups of lectins and each lectin used, stronger response to lectins in the interstitial cells (ICs) than other cell types. Moreover, the response to glucosamine (GlcNAc), galactosamine (GalNAc), and fucose tended to be higher than glucose and galactose. Furthermore, the intensity of response was increased toward the degenerative zone. In addition, we can use peanut agglutinin (PNA) as an acrosomal marker in combination with other marker proteins for studying shark spermatogenesis. These findings refer to the crucial role of glycoconjugates in spermatogenesis in the bamboo shark testis.