I. INTRODUCTION & BACKGROUND
According to the U.S. National Air and Space Administration (NASA), the level of CO2 in the atmosphere has exceeded 400 ppm, the highest it has been in over 400,000 years. 1 At the same time, over 1 billion people, primarily in sub-Saharan Africa and Asia, do not have access to electricity. These environmental and human development factors lead to a pressing need for grid energy storage to enable the integration of sustainable energy conversion devices, which are typically intermittent in nature. In addition, portable electronics and electric vehicles continue to proliferate and push the performance of lightweight, high volumetric energy density storage devices. For these diverse applications, electrochemical energy storage is the primary energy storage technology due to the large number of chemistries, their scalability, and efficiency. In addition to the large application demand, the constantly evolving capability to understand phenomena at electrochemical interfaces is leading to significant improvements in the fundamental understanding of electrochemical processes. Electrochemical measurements have now been coupled with such advanced materials characterization techniques as transmission electron microscopy (TEM), Reference Liu and Huang2–Reference Wang, Xu, Liu, Zhang, Saraf, Arey, Choi, Yang, Xiao, Thevuthasan and Baer4 x-ray diffraction (XRD), Reference Kodama, Terada, Nakai, Komaba and Kumagai5–Reference Sharma, Pang, Guo and Peterson7 x-ray absorption (XAS), Reference Mansour, Smith, Baker, Balasubramanian and McBreen8,Reference Song, Cheng, Chen, Qin, Nam, Xu, Yang, Bongiorno, Lee, Bai, Tyson, Cho and Liu9 atomic force microscopy (AFM), Reference Bearinger, Orme and Gilbert10–Reference Bullard and Smith12 and Raman microscopy, Reference Lei, McLarnon and Kostecki13–Reference Meller, Menzel, Fic, Gastol and Frackowiak15 to name just a few. This pairing has enabled in situ and in operando characterization of materials for electrochemical technologies leading to advancements in the mechanistic understanding of interfacial phenomena. Lastly, advancements in materials synthesis are leading to the control of materials at the atomic scale Reference Wu, Guo, Zheng, Xie and Stucky16–Reference Nicolosi, Chhowalla, Kanatzidis, Strano and Coleman18 so that electrochemical energy storage electrodes can be highly tailored for diverse applications, from small sensors Reference Pech, Brunet, Durou, Huang, Mochalin, Gogotsi, Taberna and Simon19,Reference Cheah, Perre, Rooth, Fondell, Hårsta, Nyholm, Boman, Lu, Simon and Edstro20 to grid energy storage. Reference Liu, Zhang, Yang, Lemmon, Imhoff, Graff, Li, Hu, Wang, Xiao, Xia, Viswanathan, Baskaran, Sprenkle, Li, Shao and Schwenzer21 With this congruence of societal need, improved understanding of interfaces, and control over material synthesis, it is no wonder that the present time is being hailed as the ‘golden age’ for electrochemistry. Reference Penner and Gogotsi22
Within this dynamic research and application landscape, layered transition metal oxides are a highly important class of materials for electrochemical energy storage due to their use in lithium ion battery cathodes, Reference Manthiram, Vadivel Murugan, Sarkar and Muraliganth23 sodium ion battery cathodes, Reference Kundu, Talaie, Duffort and Nazar24 and electrochemical capacitors. Reference Augustyn, Simon and Dunn25 The unique feature of these materials is the presence of an interlayer region that serves as the host for ion intercalation. The purpose of this review is to describe methods by which the interlayer of layered metal oxides can be tuned to achieve improvements in electrochemical performance or for new mechanisms of energy storage. The interlayer modifications discussed are the presence of structural water, solvent cointercalation and exchange, cation exchange, polymers, and small molecules, exfoliation, and synthesis of exfoliated metal oxide heterostructures.
II. STRUCTURE OF LAYERED TRANSITION METAL OXIDES
The wide interlayer spacing and weak interlayer bonding of layered oxide materials allows for the intercalation of a large variety of guest species, including cations, anions, and polymers. These layered structures are built up of transition metal–oxygen clusters, with the transition metal typically in octahedral, or 6-fold, coordination by oxygen ligands. Reference Bunker and Casey26 In layered oxides, the strong interaction between the transition metal cation and the electrons of the oxygen ligand means that oxygens will bond weakly to transition metals in adjacent layers. Reference Bunker and Casey26 The octahedra are assembled into extended structures by sharing corners, edges, and rarely, faces. The critical feature of layered transition metal oxides is that the intralayer bonding is significantly stronger than the interlayer bonding. The layered oxides are typically formed by transition metals in high oxidation states—+4, +5, and +6. Figure 1 illustrates the layered structure of several different redox-active layered oxides: (a) layered birnessite MnO2 (δ-MnO2), (b) orthorhombic V2O5, and (c) monoclinic WO3·2H2O. These represent the variety of layered structures built up from MO6 octahedra sharing edges and corners. Layered oxide structures are extremely versatile and allow for modification of both the inorganic framework (via substitutional doping Reference Johnson, Li, Lefief, Vaughey and Thackeray27 or vacancy formation) Reference Li, Wang, An, Ren, Rong and Yao28 and the interlayer; the latter is the focus of this review.

FIG. 1. Examples of layered transition metal oxides for electrochemical energy storage: (a) birnessite (δ) MnO2, (b) orthorhombic V2O5, and (c) monoclinic WO3·2H2O.
III. MECHANISMS OF ELECTROCHEMICAL ENERGY STORAGE IN LAYERED TRANSITION METAL OXIDES
The development of high energy density electrochemical storage is in large part due to the properties of layered transition metal oxides. These include high ionic and electronic conductivity, capability of undergoing redox reactions, and the availability of interlayer sites for the intercalation of cations from the electrolyte. In general, when a layered metal oxide is placed in contact with an electrolyte, several different mechanisms are possible. In order of increased capacity and structural disorder, these are: (i) double-layer capacitance, Reference Conway29 (ii) pseudocapacitance, Reference Augustyn, Simon and Dunn25 (iii) intercalation, Reference Whittingham30 (iv) decomposition, Reference Benedek, Vaughey and Thackeray31 and (v) conversion, Reference Poizot, Laruelle, Grugeon, Dupont and Tarascon32 as illustrated in Fig. 2. Double-layer capacitance is the only mechanism that is purely electrostatic and therefore does not contain a charge transfer step. Reference Conway and Pell33 As a result, this mechanism provides lifetimes of over 1 million cycles, which are orders of magnitude greater than with mechanisms that involve a redox process. The highest capacitances of ∼150 F/g are obtained with high surface area (>1000 m2/g) carbon materials. Reference Simon and Gogotsi34 Two different types of Faradaic capacitance (pseudocapacitance) can occur in transition metal oxides: redox pseudocapacitance and intercalation pseudocapacitance. Reference Conway35 Redox pseudocapacitance occurs due to surface or near-surface redox reactions; materials that exhibit this phenomenon include RuO2·0.5H2O (Ref. Reference Zheng, Cygan and Jow36) as well as most nanostructured transition metal oxides. Reference Wang, Polleux, Lim and Dunn37 Intercalation pseudocapacitance occurs due to redox reactions at the surface as well as the bulk that are not kinetically limited by solid-state diffusion or phase transitions, as in Nb2O5. Reference Augustyn, Come, Lowe, Kim, Taberna, Tolbert, Abruña, Simon and Dunn38 When present, both pseudocapacitive mechanisms will exhibit capacitive, or surface-limited, kinetics, Reference Augustyn, Simon and Dunn25 with capacitance values between ∼300 and 1000 F/g. Intercalation reactions encompass a more general mechanism than intercalation pseudocapacitance, in that kinetics can be limited by solid-state diffusion and nucleation of a new phase can occur. Intercalation reactions provide capacities of up to 400 mA h/g in the case of multi-electron intercalation. Reference Chernova, Roppolo, Dillon and Whittingham39 Decomposition and conversion reactions destroy the layered transition metal oxide structure during the 1st cycle, and require the nucleation of Li2O and suboxides or metal nanoparticles. Reference Tarascon, Grugeon, Morcrette, Laruelle, Rozier and Poizot40 These reactions provide the highest capacities for energy storage with metal oxides, typically >500 mA h/g. Reference Poizot, Laruelle, Grugeon, Dupont and Tarascon32,Reference Ban, Wu, Gillaspie, Chen, Yan, Blackburn and Dillon41 The reversibility and rate capability of decomposition and conversion reactions is high after the first cycle, but due to the variation in reaction pathways between the lithiation/delithiation processes, the overall energy efficiency is low (typically, ∼1 V hysteresis occurs even at low rates in oxides). Reference Cabana, Monconduit, Larcher and Palacín42,Reference Poizot, Laruelle, Grugeon and Tarascon43 Despite many years of intense effort to overcome this issue by designing highly advanced electrode architectures, it has been extremely challenging to improve the energy efficiency sufficiently for application in commercial devices. Reference Palacín, Simon and Tarascon44
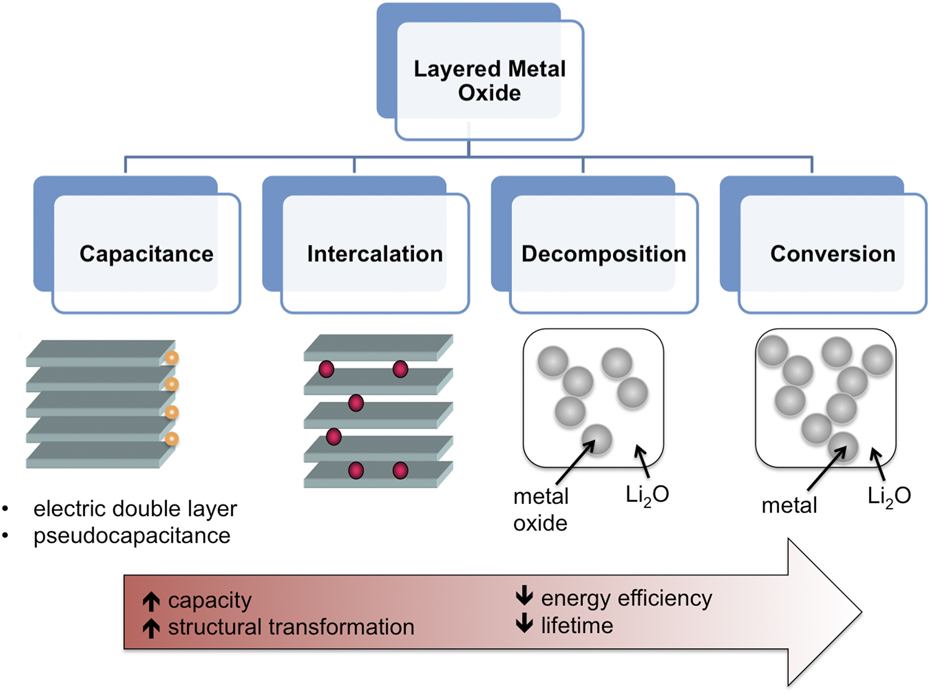
FIG. 2. Possible mechanisms of electrochemical energy storage in layered transition metal oxides. From left to right, the capacity (total amount of charge stored) increases, but so does the level of structural transformation, which results in decreasing energy efficiency and lifetime.
Intercalation is the best mechanism for energy storage in metal oxides when considering the optimization of capacity, rate capability, efficiency, and lifetime. The general intercalation equation is:
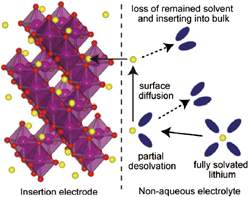
where MO is a layered transition metal oxide and A+ is a cation (typically H+, Li+, and Na+ but multivalent ions such as Mg2+ and Al3+ can also intercalate). As a result of the beneficial properties of intercalation, the mechanism has been successfully utilized for high energy density electrochemical energy storage in commercially-available lithium-ion and nickel-metal hydride batteries (Ni/MH). Reference Cheng, Liang, Tao and Chen45 The intercalation mechanism occurs via four primary steps (Fig. 3) Reference Bruce and Saidi46 : (i) ion diffusion in the electrolyte to the electrode/electrolyte interface, (ii) surface diffusion of the ion to an intercalation site, (iii) charge transfer at the interface, and (iv) diffusion in the solid state. Each of these steps can be affected by the properties of the interlayer in layered transition metal oxides.

FIG. 3. The four primary steps of ion intercalation into a solid host, illustrated for Li+ insertion from a non-aqueous electrolyte. Reprinted with permission from Ref. Reference Okumura, Fukutsuka, Matsumoto, Orikasa, Arai, Ogumi and Uchimoto47. Copyright 2011 American Chemical Society.
IV. BENEFITS OF INTERLAYER MODIFICATION FOR ELECTROCHEMICAL ENERGY STORAGE
Interlayer modification presents an additional level of control over the electrochemical behavior of oxides for electrochemical energy storage based on intercalation reactions. At the interface, interlayer modification of layered structures can affect the activation energy and potential for charge transfer. In the bulk, it may affect the electronic conductivity of the structure if the interlayer modification involves the intercalation of cations to form a partially reduced compound. Interlayer modification will affect the transport of the intercalating ion, and may alter the ion storage sites. In this section, the effect of interlayer modification on interfacial charge transfer and diffusion is presented in detail.
A. Interfacial charge transfer
The charge-transfer stage is a critical step where an ion transfers from a (typically) liquid phase into a solid phase at the same time that an electron is transferred into the solid phase. When transferring from a liquid electrolyte, the ion typically needs to lose its solvation shell Reference Xu48 and, in the case of multivalent cations, other ions. Reference Wan, Perdue, Apblett and Prendergast49 In theoretical descriptions of charge transfer, an intermediate, transient step is assumed between the solvated stage and the intercalated stage. The difference in energy between the solvated stage and the transient stage is the activation energy for charge-transfer during intercalation (E a), and may be related to the charge transfer resistance (R CT) Reference Xu48 :
Here, A 0 is a pre-exponential constant, k is the ideal gas constant, and T is the temperature. The activation energy for charge transfer has been ascribed as the energy required to desolvate the intercalating cation. Reference Xu48 Modification of the interlayer that allows for solvent co-intercalation or partial cation solvation in the structure can thus decrease the activation energy for charge transfer.
B. Ion transport in the interlayer
Once the ion has been intercalated, it undergoes solid-state diffusion due to the concentration gradient developed between the surface and the bulk of the material. Typically, diffusion is the rate-limiting step for ion intercalation in electrochemical energy storage Reference Park, Zhang, Chung, Less and Sastry50 except in the case of intercalation pseudocapacitance, which is surface limited. Reference Augustyn, Come, Lowe, Kim, Taberna, Tolbert, Abruña, Simon and Dunn38 In most layered compounds, the ions move via interstitial vacancies in a 2D plane. In a layered oxide with a modified interlayer, the ion may diffuse via different mechanisms. For example, in a hydrated, layered structure, the ion may move via the Grotthus mechanism responsible for rapid proton diffusion in water. Reference Whittingham51 The use of interlayer solvation in multivalent ion intercalation is beneficial for screening the diffusing ion from the inorganic framework. Reference Nam, Kim, Lee, Salama, Shterenberg, Gofer, Kim, Yang, Park, Kim, Lee, Chang, Doo, Jo, Jung, Aurbach and Choi52,Reference Song, Gillette, Lee, Noked, Rubloff, Gillette, Duay, Rubloff and Lee53
The interlayer environment can lead to changes in the rate capability for ion intercalation due to its influence on the diffusion coefficient, D Reference Van der Ven, Bhattacharya and Belak54 :
where p is a geometrical factor related to the interstitial vacancy network (1D, 2D, or 3D), λ is the hopping distance, v* is the vibrational frequency, and E B is the activation barrier for hopping, or the maximum energy point between the initial and final site of the diffusing ion. It is this activation barrier that will be affected by changes due to the interlayer environment, including the presence of interlayer molecules. As in the case of the activation energy barrier for charge transfer, the exponential dependence of the diffusion coefficient on E B indicates that even small changes in the diffusion environment will lead to significant changes in the diffusion coefficient.
V. INTERLAYER MODIFICATION OF LAYERED METAL OXIDES
Layered oxides form because of strong, directional bonding between oxygen and transition metal ions that results in the formation of weakly bonded atoms separated by large bonding distances. Therefore the general characteristic of layered structures is that there exists strong, covalent-ionic bonding within layers and weak, van der Waals bonding between layers. The interlayer binding energy for most layered compounds has been shown to be ∼20 meV/Å2 by computational methods. Reference Bjorkman, Gulans, Krasheninnikov and Nieminen55 In comparison, the cleavage energy for a nonlayered compound such as NiO was calculated to be 333 meV/Å2, Reference Wander, Bush and Harrison56 or approximately 16 times the energy of the interlayer bonding. In oxides, individual layers are negatively charged Reference Schöllhorn, Kuhlmann and Besenhard57 so the interlayer may be modified by charged or polar species. The interaction between the interlayer species and the layers can be weak, so the species themselves can be fairly mobile and thus removed via solvent exchange (the application of a concentration gradient) or, if they are charged, by application of an electric field. This section will highlight the means by which the interlayer spacing of layered oxides can be modified and the effect this has on the electrochemical energy storage properties.
A. Structural water
Interlayer water molecules can be present within a layered structure by three different means: (i) as structural water present in the as-synthesized oxide; (ii) as a result of water diffusion into the interlayer spacing; and (iii) via electrochemical cycling in aqueous electrolytes. This section will focus on layered oxides with structural water, which include WO3·nH2O, Reference Freedman58 MoO3·nH2O, Reference Kuzmin and Purans59 V2O5·nH2O, Reference Augustyn and Dunn60 and birnessite (δ) MnO2. Reference Ghodbane, Pascal and Favier61 In particular, the molybdenum and tungsten oxides form a series of stable and metastable hydrous phases. The stable phases include monoclinic WO3·2H2O, WO3·H2O, MoO3·2H2O, and MoO3·H2O. The dihydrates and monohydrates of tungsten and molybdenum are isostructural; the structures of the tungsten oxide hydrates are shown in Fig. 4. These hydrates are built up of corner-sharing and tilted octahedra. In the case of the monohydrate, the water is located at the apex of the octahedra whereas in the dihydrate, the second water is hydrogen bonded within the interlayer.

FIG. 4. Relationship between crystal structure and temperature of WO3 hydrates. MoO3 hydrates are isostructural with the tungsten oxide hydrates.
The effect of structural water on electrochemical energy storage has been investigated with molybdenum oxide hydrates cycled in nonaqueous lithium ion electrolytes. Nazri et al. Reference Yebka, Julien and Nazri62 reported that molybdenum oxide hydrates (MoO3·H2O and MoO3·2/3H2O) exhibited higher capacity and better cyclability than anhydrous MoO3. Kumagai et al. Reference Kumagai, Kumagai and Tanno63 reported that the Li+ intercalated into the structure in between the hydrated layers, and, in the monohydrate, obtained capacities of up to 400 mA h/g at a current density of 0.2 mA/cm2 between 3 and 1 V versus Li/Li+. Due to the potential range for intercalation (3.5–1.5 V versus Li/Li+) and lack of Li in the as-synthesized structure, the materials are best suited as cathodes for primary batteries or anodes for hybrid electrochemical capacitors.
In aqueous electrolytes, interlayer water molecules have been hypothesized to provide rapid diffusion channels for protons. The Grotthus mechanism of proton diffusion Reference Whittingham51 has been proposed in hydrated tunnel structures, such as those formed by hexagonal MoO3, and is expected to occur in layered structures as well. Reference Muñoz-Santiburcio, Wittekindt and Marx64 This mechanism accounts for the rapid diffusion of protons in aqueous electrolytes via the formation and deformation of hydrogen bonds on water molecules. Reference Winter and Brodd65 However, it is not clear whether the Grotthus mechanism occurs in all hydrated layered oxides. Recent density functional theory calculations indicated that protons do not intercalate via the water network in WO3·2H2O; instead, it was theorized that the mechanism of proton intercalation is binding to a bridging oxygen, the same as in WO3. Reference Lin, Zhou, Liu and Ozolins66 On the other hand, experimental results of electrochromic hydrated tungsten oxides show rapid coloration and bleaching in acidic electrolytes, which was ascribed to rapid proton diffusion via the Grotthus mechanism. Reference McIntyre, Basu, Peck, Brown and Augustyniak67 The room temperature proton conductivity at ∼50% relative humidity of WO3·nH2O was determined to be ∼1 × 10−5 S/cm for the dihydrate and monohydrate, and the proton conduction was hypothesized to occur via the interlayer hydrogen bonded network as in the Grotthus mechanism. Reference Li, Hibino, Miyayania and Kudo68 These diverse results underscore the need for in-depth understanding of transport mechanisms during electrochemical intercalation in hydrated structures.
In addition to the potential for rapid proton diffusion via the Grotthus mechanism, the presence of interlayer water molecules has been correlated with improved intercalation kinetics of multivalent ions. Multivalent ions are those with a formal charge greater than 1; of particular interest to energy storage applications is the storage of the divalent cation Mg2+. This is because Mg metal, with a theoretical capacity of 2205 mA h/g (for the reaction Mg2+ + 2e− ↔ Mg) Reference Walter, Kravchyk, Ibáñez and Kovalenko69 can be reversibly cycled in a suitable nonaqueous Mg-ion electrolyte without the formation of dendrites, as in the case of lithium metal. Thus the use of Mg metal as an anode allows for achieving both high energy density and safety. One of the challenges of Mg batteries, however, is finding a high voltage and high capacity cathode material. Intercalation of Mg2+ is not as facile as Li+ due to the sluggish solid state diffusion of Mg2+, which is due to (i) the Coulombic repulsion between the framework transition metals and the diffusing Mg2+ ions, (ii) the need for a transition metal to accept 2e−, which results in an increase in the ionic radius and thus unit cell volume, Reference Levi, Levi, Chasid and Aurbach70 and (iii) the strong solvation Reference Lapidus, Rajput, Qu, Chapman, Persson and Chupas71 and anion coordination Reference Wan, Perdue, Apblett and Prendergast49 of Mg2+, which raises the activation energy for charge-transfer at the electrode/electrolyte interface.
The presence of interlayer water molecules can enable the reversible intercalation of Mg2+ because these species can act as a solvation shell, and shield the diffusing divalent cation from the lattice anions and cations. Reference Levi, Gofer and Aurbach72 For example, V2O5 aerogels are high-surface area oxide materials made via supercritical drying of a V2O5 gel. Reference Chaput, Dunn, Fuqua and Salloux73,Reference Le, Passerini, Guo, Ressler, Owens and Smyrl74 Their short-range structure is similar to that of V2O5 xerogels, Reference Petkov, Trikalitis, Bozin, Billinge, Vogt and Kanatzidis75 which consists of V2O5 bilayers separated from each other by a large interlayer spacing (∼11.5 Å) filled with water molecules. In the case of the aerogels, the nominal formula after supercritical drying is V2O5·2H2O and ∼1.5 H2O molecules are removed by heat treatment at 120 °C. Reference Chaput, Dunn, Fuqua and Salloux73 The maximum amount of intercalation in the aerogel is ∼0.6 Mg2+ per V2O5 Reference Tang, Sakamoto, Baudrin and Dunn76,Reference Le, Passerini, Coustier, Guo, Soderstrom, Owens and Smyrl77 which corresponds to a gravimetric capacity of 88 mA h/g. Figure 5(b) shows the cyclic voltammogram of a V2O5·0.5H2O aerogel in Mg(ClO4)2 in propylene carbonate electrolyte. It should be noted that while Mg2+ intercalation is reversible in V2O5 aerogels, the separation between the cathodic (Mg2+ intercalation) and anodic (Mg2+ deintercalation) peaks is larger than in the case of Li+ intercalation [Fig. 5(a)], indicating that kinetics are still sluggish despite the benefit of interlayer water.
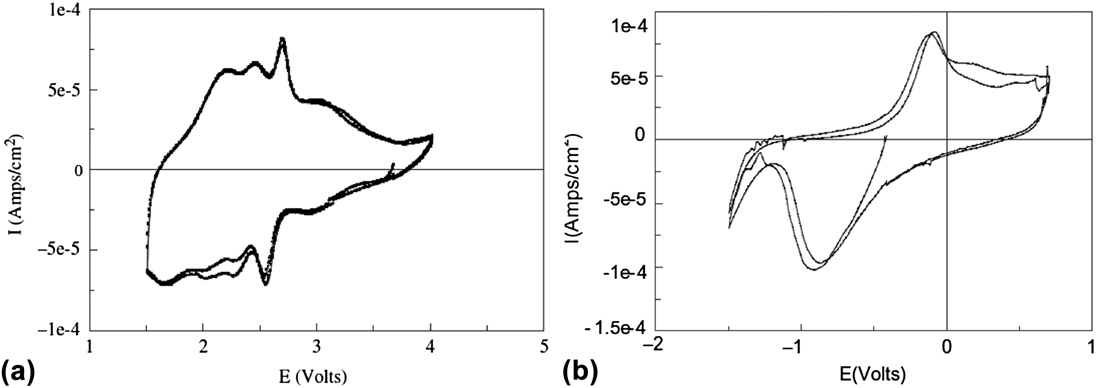
FIG. 5. Cyclic voltammetry of V2O5·0.5H2O aerogel in (a) LiClO4 in propylene carbonate and (b) Mg(ClO4)2 in propylene carbonate electrolytes at 0.1 mV/s. The aerogel can be reversibly cycled in both electrolytes due to the nanostructured morphology and presence of structural water. Reprinted from Ref. Reference Tang, Sakamoto, Baudrin and Dunn76, with permission from Elsevier.
One potential drawback of structural water is that it may be removed during electrochemical cycling, leading to changes in the energy storage behavior, crystal structure, and potential contamination of a non-aqueous electrolyte with water. Reference Levi, Gofer and Aurbach72 The significance of this issue on the stability of interlayer structural water during electrochemical cycling varies. In the case of V2O5 aerogels, Le et al. Reference Le, Passerini, Coustier, Guo, Soderstrom, Owens and Smyrl77 used chemical analysis to determine that structural water was retained after chemical and electrochemical intercalation of Li+ and Mg2+. On the other hand, Novák et al. Reference Novák, Scheifele, Joho and Haas78 reported that structural water was removed upon repeated electrochemical intercalation and deintercalation of Mg2+ in hydrated layered vanadium oxide bronzes of the family MV3O8·nH2O, where M = Li, Na, K, Ca0.5, and Mg0.5, and this group reported the same water loss in V2O5 xerogel. The decrease in structural water content was correlated with decreased capacity for Mg2+ in the materials. Recently, the decrease in capacity of V2O5·0.6H2O with cycling in a Li+ nonaqueous electrolyte was correlated with accumulation of LiOH. Reference Wangoh, Huang, Jezorek, Kehoe, Watson, Omenya, Quackenbush, Chernova, Whittingham and Piper79 In the case of MoO3·nH2O cycled in a nonaqueous Li+ electrolyte, the decrease in capacity with cycling of anhydrous MoO3 was greater than that of the hydrous phases, MoO3·H2O and MoO3·1/3H2O. Reference Yebka, Julien and Nazri62 Kumagai et al. Reference Kumagai, Kumagai and Tanno63 proposed that electrochemically intercalated Li+ react with the interlayer water molecules of MoO3·2H2O because of the observed poor reversibility and decrease in interlayer spacing upon Li+ intercalation from ex situ XRD; the monohydrate was more stable. Based on these reports, it is possible that the removal and reactivity of interlayer water is more likely in structures where the water molecules are bound via hydrogen bonds to the interlayer, and not covalently bound as in the case of MoO3·H2O.
B. Solvent cointercalation & exchange
The previous section discussed the behavior of layered crystal structures with interlayer water molecules present in the as-synthesized state. For other layered structures, water and other polar solvent molecules may be intercalated during electrochemical cycling (so-called co-intercalation) or by solvent exchange. Reference Schöllhorn, Kuhlmann and Besenhard57 Adapting the nomenclature established by Schöllhorn Reference Schöllhorn, Whittingham and Jacobson80 these reactions can be written as:
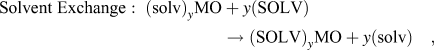 $${\rm{Solvent}}\;{\rm{Exchange}}:\;{\left( {{\rm{solv}}} \right)_y}{\rm{MO}} + y\left( {{\rm{SOLV}}} \right) \to {\left( {{\rm{SOLV}}} \right)_y}{\rm{MO}} + y\left( {{\rm{solv}}} \right)\quad ,$$
$${\rm{Solvent}}\;{\rm{Exchange}}:\;{\left( {{\rm{solv}}} \right)_y}{\rm{MO}} + y\left( {{\rm{SOLV}}} \right) \to {\left( {{\rm{SOLV}}} \right)_y}{\rm{MO}} + y\left( {{\rm{solv}}} \right)\quad ,$$
where MO is a layered oxide, A+ is metal cation, and SOLV and solv represent two different solvents. These reactions present an additional mechanism for the control of the interlayer of transition metal oxides for electrochemical energy storage.
Solvent cointercalation has been investigated for improving the kinetics of Mg2+ energy storage. Using first-principles calculation, Gautam et al. Reference Sai Gautam, Canepa, Richards, Malik and Ceder81 found that the cointercalation of water with Mg2+ into xerogel V2O5 in nonaqueous electrolytes can increase the intercalation potential. Previously, Levi et al. Reference Levi, Gofer and Aurbach72 proposed that the increase in potential occurs because the intercalating ion does not need to shed a solvation shell (or does so only partially) during the charge-transfer step. Solvent cointercalation can be used to synthesize electrochemically active materials, such as the transformation of spinel Mn3O4 into δ-MnO2 in aqueous electrolytes. Reference Nam, Kim, Lee, Salama, Shterenberg, Gofer, Kim, Yang, Park, Kim, Lee, Chang, Doo, Jo, Jung, Aurbach and Choi52,Reference Song, Noked, Gillette, Duay, Rubloff and Lee82 Such hydrated δ-MnO2 has been investigated as a potential cathode material for Mg ion batteries. Reference Nam, Kim, Lee, Salama, Shterenberg, Gofer, Kim, Yang, Park, Kim, Lee, Chang, Doo, Jo, Jung, Aurbach and Choi52,Reference Song, Noked, Gillette, Duay, Rubloff and Lee82,Reference Sun, Duffort, Mehdi, Browning and Nazar83 The improved intercalation kinetics and reversibility of Mg2+ in δ-MnO2 have been ascribed to efficient Coulombic screening of the intercalating Mg2+ from the host structure by interlayer water molecules [Fig. 6(a)]. First, spinel Mn3O4 was cycled in an aqueous Mg2+ electrolyte to form hydrated, layered δ-MnO2. Then, the material was cycled in a nonaqueous Mg2+ electrolyte with varying amounts of water. Compared with the completely anhydrous electrolyte, hydrated δ-MnO2 cycled in a Mg2+ nonaqueous electrolyte with small amounts of water showed higher capacity and lifetime [Figs. 6(b) and 6(c)]. However, Sun, et al. found that the hydrated material undergoes a conversion reaction with Mg2+ in a nonaqueous electrolyte with the formation of MnOOH, MnO, and Mg(OH)2; reversible intercalation was only observed in an aqueous Mg2+ electrolyte. Reference Sun, Duffort, Mehdi, Browning and Nazar83

FIG. 6. (a) Proposed mechanism for the enhanced electrochemical energy storage of Mg2+ in layered δ-MnO2. Reproduced from Ref. Reference Song, Noked, Gillette, Duay, Rubloff and Lee82 with permission of the PCCP Owner Societies. (b) Galvanostatic charge/discharge of δ-MnO2 in Mg2+ non-aqueous electrolyte with different water content, and (c) capacity versus cycle number of the same system. Reprinted with permission from Ref. Reference Nam, Kim, Lee, Salama, Shterenberg, Gofer, Kim, Yang, Park, Kim, Lee, Chang, Doo, Jo, Jung, Aurbach and Choi52. Copyright 2015 American Chemical Society.
The benefits of water co-intercalation for the reversibility of multivalent ion intercalation are not limited to Mg2+. González et al. Reference González, Nacimiento, Cabello, Alcántara, Lavela and Tirado84 reported on the reversible charge storage of Al3+ from an aqueous electrolyte into V2O5 xerogel and proposed a co-intercalation reaction mechanism:
Since Al3+ is a small cation (ionic radius of 0.68 Å) with a large charge, it has a high standard hydration enthalpy, which means that it is very strongly hydrated. Therefore, it is reasonable to assume that solid state intercalation of Al3+ can only occur via solvent cointercalation, otherwise, the activation energy for charge transfer at the interface is too high.
Solvent exchange of water for other polar molecules can be performed in structures that contain water in the as-synthesized state or in anhydrous layered oxides whose interlayer bonding is weak enough that solvent molecules may intercalate. Molybdenum oxides serve as model systems for investigating solvent exchange because both hydrated and anhydrous crystalline MoO3 form layered structures, Reference Chippendale, Cheetham, Braithwaite and Haber85 which expands the number of precursors that can be used for solvent exchanged MoO3. Schöllhorn et al. Reference Schöllhorn, Kuhlmann and Besenhard57 performed solvent exchange of Na0.5(H2O) y MoO3 bronze with dimethylsulfoxide (DMSO); the interlayer spacing increased from 11.41 to 16.92 Å. In a related experiment, Na0.5(H2O) y MoO3 bronze was used to intercalate various organic compounds; interlayer spacings as large as 37.9 Å were reported for MoO3 intercalated with tetradecylamine. Reference Tagaya, Ara, Kadokawa, Karasu and Chiba86 Chen et al. Reference Chen, Liu, Yin, Li, Yang, Li, Fan, Wang, Zhang, Li, Xu, Lu, Yang, Sun and Gao87 utilized MoO3·H2O as the precursor for the solvent exchange of water with n-octylamines in ethanol. The reaction was described as an acid–base reaction between the water molecules and amines. The amines occupy the interlayer space in a bilayer arrangement with a 51° tilt angle and with an expanded interlayer spacing of 23.1 Å, as illustrated in Fig. 7; heat treatment of the materials at 550 °C yields anhydrous MoO3. The electrochemistry of these materials was not reported and bears further investigation as such large interlayer spacings may be beneficial for multivalent and high-rate intercalation.

FIG. 7. Synthesis of a MoO3-alkylamine hybrid starting from (a) MoO3·H2O precursor, (b) structure of the inorganic-organic hybrid including orientation of alkyl amine chains, (c) thermal heat treatment at 550 °C yields orthorhombic α-MoO3. Reproduced from Ref. Reference Chen, Liu, Yin, Li, Yang, Li, Fan, Wang, Zhang, Li, Xu, Lu, Yang, Sun and Gao87 with permission of The Royal Society of Chemistry.
C. Cation exchange
Layered metal oxides may also undergo cation exchange. In transition metal oxides, the layers are negatively charged and can thus accommodate cationic interstitial guest species. For cation exchange, the starting material may contain the exchangeable ion in the as-synthesized state (e.g., LiCoO2) or as a result of electrochemical cycling (e.g., Li x V2O5). Cation exchange may occur by the exchange of only the cation or with solvent co-intercalation, as described above. A recent review summarized the general features of cation exchange in nanoscale materials. Reference Rivest and Jain88
The desire to improve the electrochemical behavior of lithium ion cathode materials has focused significant effort on the cation exchange of layered metal oxides. LiCoO2 is a prototypical Li-ion battery cathode material whose structure consists of alternating layers of lithium and cobalt separated by oxygen layers in an …ABCABC… stacking sequence. Reference Manthiram, Nazri and Pistoia89 A low-temperature route for the synthesis of LiCoO2 and LiNiO2 was proposed by Larcher et al. Reference Larcher, Palacín, Amatucci and Tarascon90 who performed the cation exchange of NiOOH and CoOOH in a hydrothermal reactor in the presence of LiOH; Fig. 8 shows the topotactic nature of the cation exchange. The oxyhydroxides are similar in structure to the LiMO2 (M = Ni, Co). In the case of LiCoO2, the electrochemical behavior of the cation-exchanged phase was inferior to the high temperature phase due to lower coulombic efficiency. On the other hand, cation exchanged LiNiO2 exhibited similar capacity and reversibility as the high temperature analog. The increased surface area of the cation exchanged LiNiO2 did result in increased moisture sensitivity as compared to the high temperature phase, Reference Larcher, Palacín, Amatucci and Tarascon90 thus exacerbating the well-known surface instability issues of LiNiO2. Reference Liu, Oh, Liu, Lee, Cho, Chae, Kim and Cho91
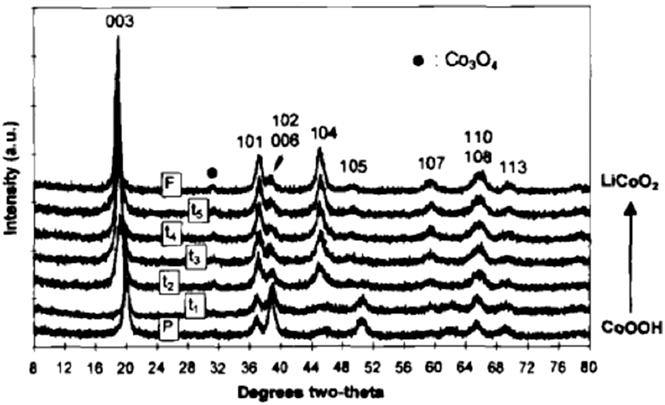
FIG. 8. XRD patterns as a function of the extent of cation exchange of CoOOH (HCoO2) to LiCoO2. Reprinted with permission from Ref. Reference Larcher, Palacín, Amatucci and Tarascon90. Copyright 1997, The Electrochemical Society.
Cation exchange is also useful as a low temperature method to synthesize metastable phases. A well-known example is the cation exchange synthesis of LiMnO2, reported by Armstrong et al. Reference Armstrong, Bruce, Armstrong and Bruce92 and Capitaine et al. Reference Capitaine, Gravereau and Delmas93 by stirring NaMnO2 in LiCl or LiBr in alcohol. However, during electrochemical cycling, the obtained LiMnO2 with an O3 layered structure undergoes conversion to a more stable spinel phase. Reference Quine, Duncan, Armstrong, Robertson and Bruce94 Therefore, one of the challenges of synthesis via cation exchange is that the structure may convert to a more stable phase during prolonged electrochemical cycling.
D. Polymers and small molecules
Some structures can exhibit very large interlayer spacings that can accommodate not just solvent molecules or cations, but polymers. Of interest for electrochemical energy storage are compounds that will increase the capacity by providing additional redox active sites or that will increase the electronic conductivity of the oxide to increase the rate capability. An example of a polymer that can provide both is polyaniline (PANI), which can be intercalated into V2O5 xerogels, with a resulting interlayer spacing of 14–19 Å. Reference Ruiz-hitzky and Ruiz-Hitzky95 As shown in Fig. 9(a), the PANI is only present within the interlayer. Figure 9(b) shows the galvanostatic charge/discharge of nanostructured V2O5 and V2O5/PANI nanocomposite; the capacity of the polymer-intercalated oxide was approximately doubled at the same time that the electronic conductivity increased ∼50 times. Reference Chen, Yang, Zhang, Yang, Hou and Zhu96
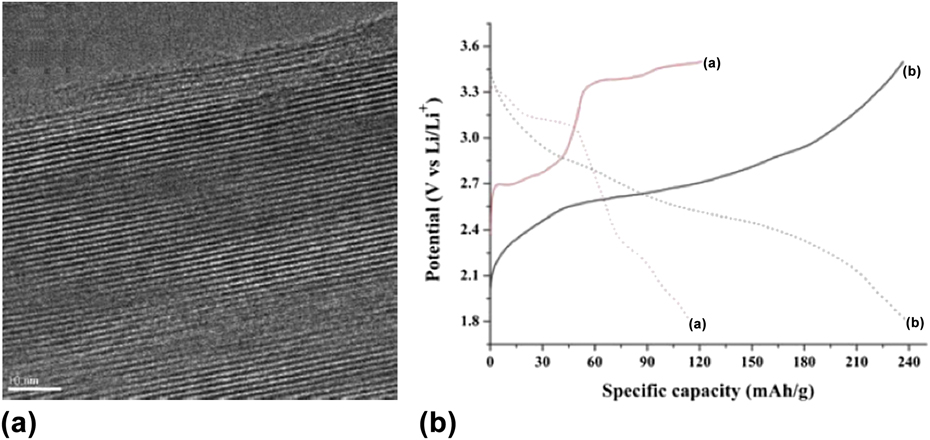
FIG. 9. (a) TEM of a vanadium oxide/polyaniline composite, where the polyaniline is inserted into the interlayer spacing of the oxide. Reprinted from Ref. Reference Malta and Torresi97, with permission from Elsevier. (b) Galvanostatic charge/discharge of nanostructured V2O5 (curve “a”) and V2O5/PANI nanocomposite (curve “b”) in a non-aqueous Li+ electrolyte at a current density of 29.5 mA/g. Reproduced from Ref. Reference Chen, Yang, Zhang, Yang, Hou and Zhu96 with permission of The Royal Society of Chemistry.
E. Exfoliation
An important property of bulk layered oxides is that they can be completely exfoliated into mono- or few-layer sheets (‘nanosheets’). There has been significant interest in discovering the electrochemical energy storage properties of such materials because, in theory, they exhibit short diffusion distances and a large number of surface redox sites Reference Peng, Zhu, Chen, Ruoff and Yu98,Reference Liu and Liu99 which could lead to high power and high energy density storage. In this regard, the methods of modifying the interlayer described above have all been utilized as a means to expand the interlayer spacing of layered oxides, which decreases the force needed to pull apart the layers into nanosheets. The applied force can be mechanical, Reference Kalantar-Zadeh, Vijayaraghavan, Ham, Zheng, Breedon and Strano100 acoustic, Reference Zhang, Gao and Gong101 thermal, Reference Du, Zheng, Zhang, Wang, Chen, Xue, Dai, Ji and Cao102 or a combination of these. Reference Aksit, Toledo and Robinson103 Figure 10 shows the general mechanism of exfoliation of layered materials into few-layer sheets. Reference Nicolosi, Chhowalla, Kanatzidis, Strano and Coleman18 A recent review described synthesis methods for controlling nanosheet size, composition, and structure. Reference Ma and Sasaki104

FIG. 10. Schematic description of exfoliation mechanisms of layered materials to yield nanosheets: (a) intercalation followed by agitation; (b) ion exchange followed by agitation; (c) direct agitation via sonication. From Ref. Reference Nicolosi, Chhowalla, Kanatzidis, Strano and Coleman18. Reprinted with permission from AAAS.
Such exfoliated layered oxide nanosheets have primarily been investigated for use as electrochemical capacitor electrodes, and specifically as pseudocapacitors. Reference Augustyn, Simon and Dunn25 This is because the nanosheet architecture would ideally expose all of the redox active sites directly to the electrolyte and enable rapid charge-transfer reactions with minimal solid state diffusion. As noted previously, MoO3 forms both hydrous and anhydrous layered polymorphs and it is a good precursor material for the synthesis of nanosheets. Bulk crystalline MoO3 can be exfoliated by sonicating a dispersion of the oxide in a solution of water and isopropanol Reference Zhang, Gao and Gong101 ; the mechanism of exfoliation is the intercalation of isopropanol between MoO3 layers and subsequent application of acoustic energy in the form of sonication. Hanlon et al. Reference Hanlon, Backes, Higgins, Hughes, O'Neill, King, McEvoy, Duesberg, Mendoza Sanchez, Pettersson, Nicolosi and Coleman105 synthesized MoO3 nanosheets via liquid-phase exfoliation that consisted of sonicating MoO3 in isopropanol. Figure 11 shows the dispersion, absorbance, and TEM of the exfoliated nanosheets separated by size into small, very small, and large; the size selection was obtained by centrifuging the stock solution at different speeds and selecting the supernatant. The maximum capacitance (∼200 F/g1 at 10 mV/s or 200 s) was obtained by combining the exfoliated MoO3 nanosheets with carbon nanotubes.

FIG. 11. Synthesis of MoO3 nanosheets via liquid exfoliation of bulk MoO3: (a) dispersions of (left to right) very small, small, and large nanosheets; (b) measurement of sedimentation via absorption as a function of time; (c) TEM of very small MoO3 nanosheets; (d) small MoO3 nanosheets; and (e) large MoO3 nanosheets. Reference Hanlon, Backes, Higgins, Hughes, O'Neill, King, McEvoy, Duesberg, Mendoza Sanchez, Pettersson, Nicolosi and Coleman105 Reprinted with permission from Ref. Reference Hanlon, Backes, Higgins, Hughes, O'Neill, King, McEvoy, Duesberg, Mendoza Sanchez, Pettersson, Nicolosi and Coleman105. Copyright 2014 American Chemical Society.
The benefits of synthesizing nanosheets from exfoliated bulk oxides is that this technique is readily scalable. Reference Nicolosi, Chhowalla, Kanatzidis, Strano and Coleman18 However, there are several challenges for using exfoliated nanosheets as energy storage materials for large-scale devices. Reference Palacín, Simon and Tarascon44 The high surface area of nanosheets can lead to increased side reactions with the electrolyte, which have to be avoided for long cycle life and safety. Second, the high surface area can also lead to low volumetric capacity/capacitance as compared with the bulk materials. Third, such materials contain many defects that can lead to limited cycle life and barriers for ion diffusion. On the other hand, nanosheets can be highly advantageous for small-scale energy storage devices. Reference Li, Shi, Lang, Wen, Li and Jiang106 Also, restacked exfoliated nanosheets have recently been reported as precursors for the synthesis of Li-ion battery cathode materials, presenting an interesting strategy for the development of metastable layered oxides. Reference Cheng, Yang, Li, Li and Chan107
F. Exfoliated metal oxide heterostructures
Exfoliated layered oxide materials can be assembled into new heterostructured layered oxide materials. Reference Geim and Grigorieva108 The motivation for synthesizing such materials is that they can exhibit improved properties over the individual components. In the case of oxide materials, this typically means improving their electronic conductivity to enable higher rate capability by forming heterostructures with graphene. One example of this method is the synthesis of graphene/MnO2 hybrids via co-exfoliation for use as electrochemical capacitors. Reference Mendoza-Sánchez, Coelho, Pokle and Nicolosi109 The highest capacitance was obtained with the co-exfoliated materials (as opposed to the individual components); a volumetric capacitance of 200 F/cm3 was obtained at a sweep rate of 100 mV/s (charge/discharge time of 10 s). The synthesis of these heterostructures opens up the possibility of entirely new metal oxide/hybrid materials assembled at the atomic layer. Most of these composites; however, are synthesized via self-assembly Reference Wang, Kou, Choi, Yang, Nie, Li, Saraf, Hu, Zhang, Graff, Liu, Pope and Aksay110,Reference Wu, Zhou, Yin, Ren, Li and Cheng111 as opposed to bulk exfoliation.
VI. FUTURE OUTLOOK & CONCLUSIONS
Layered oxides have played a key role in the development of high energy density electrochemical storage, from the investigations of alkali ion intercalation in oxides in the 1970s by Whittingham Reference Whittingham112 to the identification of LiCoO2 cathodes by Goodenough Reference Mizushima, Jones, Wiseman and Goodenough113 in the early 1980 to subsequent commercialization of high energy density lithium-ion batteries for portable electronics. In the future, layered oxides will continue to be utilized as electrodes in next generation devices including advanced lithium-ion batteries, sodium-ion batteries, and electrochemical capacitors, that take advantage of their energy density, power density, environmental stability, high potential, and lifetime. Within this class of materials, the vacant site for energy storage can be modified, and this presents a unique ‘control knob’ to tune the atomic and nanoscale electrochemical environment of bulk oxides that is not readily available in other structures.
The interlayer can be modified by the presence of structural or co-intercalated water and solvent molecules, cation exchange, or polymeric and molecules species. These can enhance the charge transfer by increasing the interlayer spacing to allow solvent co-intercalation, or for partial solvation of the intercalating ion within the interlayer. Diffusion in the bulk can be enhanced by allowing either fast transport (e.g., proton hopping via hydrogen bonding) or, in the case of multivalent ion intercalation, partial charge screening. Further research is needed to understand the exact mechanisms and lifetime of interlayer modified materials and to extend these methods from model layered oxides such as V2O5 and MoO3 to cathode materials such as LiCoO2. There has been some recent promising work in this area for nonaqueous sodium-ion batteries. For example, interlayer modification of layered sodium metal oxide cathodes by doping of the interlayer with small amounts of Li Reference Kim, Kang, Slater, Rood, Vaughey, Karan, Balasubramanian and Johnson114 or Mg Reference Singh, Tapia-ruiz, Miguel, Maitra, James, Armstrong, De Ilarduya, Rojo, Bruce, Singh, Tapia-ruiz, Miguel, Maitra, So, Armstrong, De Ilarduya, Rojo and Bruce115 improves the cycling stability by stabilizing the structure. The continuing need for better electrochemical energy storage, coupled with improvements in characterization capability and synthesis techniques, will push the properties of layered metal oxides forward. The methods of interlayer modification are an important design tool in the development of these advanced materials.
ACKNOWLEDGMENT
The author acknowledges start up funding from North Carolina State University.