Published online by Cambridge University Press: 14 July 2014
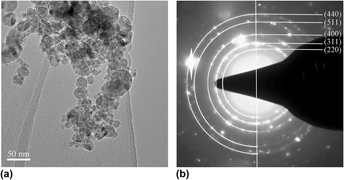
Oxide-dispersion-strengthened (ODS) ferritic alloys are fascinating materials for future high temperature energy production technologies. MgAl2O4 ODS alloy incorporating nanoscale oxide particles were produced by mechanical milling (MM) followed by hot isostatic pressing (HIP). The MgAl2O4 nanoscale oxide particles were formed during the HIP process by the addition of MgO and Al2O3 to the Fe–Cr matrix. Microstructural evolution of ODS alloys was structurally characterized at each step of the elaboration processes by means of scanning electron microscope (SEM), transmission electron microscope (TEM), and x-ray diffraction (XRD). The observations of structure of the mixed powders in ODS alloys after MM indicated that the initial powders, coupled with the original MgO and Al2O3 powders, got fractured by severe plastic deformation and ultrafine bcc grains (∼17 nm) of the matrix and amorphous phase composed of Mg, Al, and O were formed during MM. The main driving force for the formation of amorphous phase comes from the increase of volume fraction of bcc Fe grain boundary and the increase of interfacial energy due to the decrease in the size of MgO and Al2O3 powders. The MgAl2O4 nanoscale oxide particles formed at 1173 K which was far below the traditional sintering temperature of the raw material. And the structures of MgAl2O4 nanoscale oxide particles were observed by TEM.