Introduction
Nasal polyps, characterised by mucosal inflammation, are benign mucosal protrusions that expand towards the luminal surface of the nasal cavity. They are the most common type of nasal mass and are smooth and gelatinous structures that are attached to the mucosal membrane by a thin stalk or pedicle.Reference Brain, Settipane, Lund, Berstein and Tos1 The frequency of observing nasal polyps is approximately 1–4 per cent, and the condition is 2–4 times more widespread in men than women.Reference Dingsør, Kramer, Olsholt and Søderstrøm2, Reference Bateman, Fahy and Woolford3 Although many theories have been suggested with regard to their aetiopathogenesis, no firm conclusion has as yet been reached. However, it is believed that allergic, infectious, mechanical, immunological and biochemical factors play a role.Reference Bateman, Fahy and Woolford3 Histopathologically, mucosal swelling induced by inflammation is caused by basal membrane thickening, atypical gland formation, goblet cell hyperplasia, inflammatory cell infiltration and subepithelial oedema.Reference Hellquist4
The viral aetiology of sinonasal polyps was evaluated for the first time in 1966 by Weille.Reference Weille5 Although it is believed that viruses play a role in the formation of polyps, this has not been proven in studies on adenovirus, Epstein–Barr virus (EBV), herpes simplex virus and human papillomavirus (HPV).Reference Tos, Larsen, Kennedy, Bolger and Zinreich6 This study aimed to identify the role of human adenovirus, metapneumovirus, coronavirus, parainfluenza virus type 1, 2 and 3, influenza A and B virus, respiratory syncytial virus A and B and rhinovirus A and B on nasal polyp aetiology using polymerase chain reaction technique.
Materials and methods
Patient characteristics
All patients were enrolled in the study after approval from the Ethics Board of the Faculty of Medicine of Bezmiâlem Vakif University had been obtained. All patients participating in the study gave informed oral consent. All patients and control group members were questioned with regard to parameters such as age, gender, smoking status, drinking habits, asthma, aspirin intolerance, chronic obstructive pulmonary disease (COPD) and previous surgery.
Patients complaining of sneezing, rhinorrhoea, headache, nasal obstruction and hypoxaemia or anosmia and who were identified as having nasal polyps during endoscopic examination were enrolled in the study. Subjects with nasal polyps were recommended not to use any kind of medical treatment including systematic and topical steroids. Patients who were not diagnosed with any problems, excluding septum deviation, were enrolled in the control group. Subjects with systematic illness, malignancy, immune suppression, diabetes mellitus, recurrent nasal polyposis, and granulomatous and ciliary disorders were excluded from the study. Mucosa was obtained from the polyps of patients with nasal polyposis and the middle turbinate of the control group patients by means of biopsy. Samples were stored at −80 °C until molecular analysis with polymerase chain reaction was carried out.
Application of polymerase chain reaction
We used the Seeplex RV12 ACE Detection Kit (Seegene, Seoul, South Korea) to detect the viruses. Seeplex RV12 ACE detection is based on four major processes: nucleic acid isolation; reverse transcription; polymerase chain reaction amplification of target DNA using dual-priming oligonucleotide primers; and DNA detection with agarose gel electrophoresis.
Nucleic acids were isolated and reverse transcription was applied using the RevertAid First Strand cDNA Synthesis Kit (Fisher Scientific, Loughborough, UK), then the cDNA of the samples were amplified by means of polymerase chain reaction using the Seeplex RV12 ACE Detection Kit protocol. Deoxyribonucleic acid concentration was determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware, USA). Negative and positive controllers suitable for each polymerase chain reaction were used to avoid contamination and to determine the specificity of primer-directed amplification.
The polymerase chain reaction product of each sample (5 μl) and marker (5 μl) were electrophoresed on 2 per cent agarose gel containing ethidium bromide, and were photographed with an ultraviolet light illuminator. The sensitivity of polymerase chain reaction analysis was determined using amplification tests on serial dilutions of the viral-positive control DNA. Analysis of the positive samples was repeated to ensure accuracy.
Statistical analysis
Pearson's correlation coefficient with two variables or the non-parametric Kruskal–Wallis H tests were used for statistical analysis. P values less than 0.05 were considered to be statistically significant. The entire analysis was carried out using SPSS version 16.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
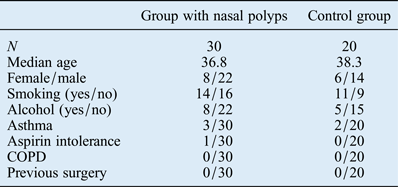
Thirty patients with nasal polyps (22 men and 8 women; average age: 36.8 years; age range: 21–67 years) and a control group of 20 healthy patients (14 men and 6 women; average age: 38.3 years; age range: 18–72 years) were included in this study. The two groups were comparable in terms of age, female/male ratio, smoking status, drinking habits, asthma, aspirin intolerance, COPD and previous surgery (Table I). Nasal polyposis was diagnosed histopathologically in 30 patients.
Table I Clinicopathological characteristics of the two study groups

COPD = chronic obstructive pulmonary disease
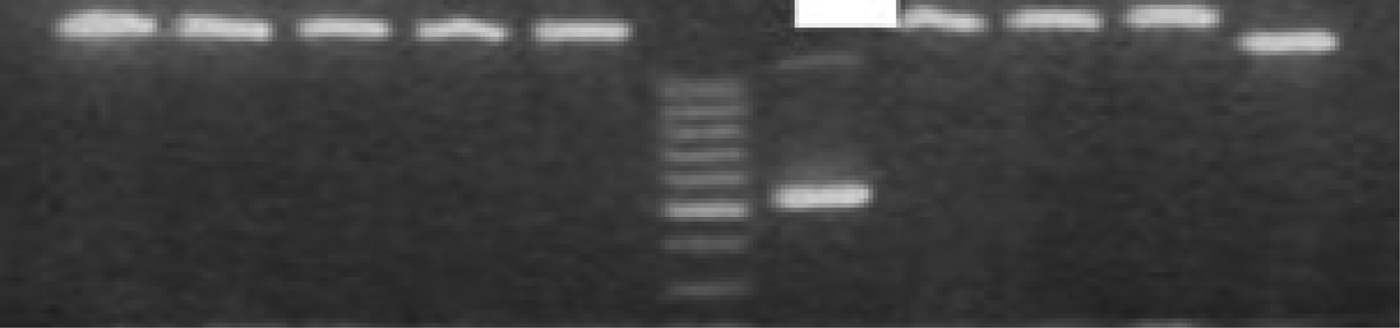
The tissues were examined by agarose gel electrophoresis after polymerase chain reaction amplification. Human coronavirus and rhinovirus were diagnosed in one of the patients in the control group (20 patients) and human respiratory syncytial virus was identified in another control group patient, while no viral pathogen was identified in the remaining 18 patients in the control group. The influenza B virus was identified in one of the nasal polyposis patients and human coronavirus was identified in another patient (Figure 1). No viral pathogen was identified in any of the remaining 28 patients. The results did not demonstrate a statistically significant relationship between nasal polyposis and respiratory viruses in either group (p < 0.001; Pearson's correlation coefficient with two variables).

Fig. 1 The agarose gel electrophoresis of amplified products yielded one positive sample for human coronavirus OC43/HKU1 (578 bp) in patients with nasal polyposis.
Discussion
Nasal polyposis in the nose or paranasal sinuses is characterised by stromal oedema causing chronic inflammation.Reference Bateman, Fahy and Woolford3 Although the incidence of nasal polyposis among adults in the USA and Europe is 4.3 per cent, the cause of the condition has not yet been identified. It has been suggested that many exogenic and endogenic factors result in the formation of nasal polyps, including bacterial and viral infections.Reference Watelet, Claeys, Perez-Novo, Gevaert, Van Cauwenberge and Bachert7–Reference Little, Early, Woodard, Shonka, Han and Borish10
In this study, conducted using polymerase chain reaction, known to be a reliable technique, we concluded that there was no association between nasal polyps and viral infections, which is compatible with the existing English language literature.Reference Hoffmann, Klose, Gottschlich, Görögh, Fazel and Lohrey11
Although the prevalence of nasal polyps is 1–4 per cent,Reference Dingsør, Kramer, Olsholt and Søderstrøm2, Reference Bateman, Fahy and Woolford3 in one endoscopic autopsy study, it was shown that this value may increase up to 32 per cent.Reference Larsen and Tos12 Nasal polyposis affects adults in the majority of cases and it is generally observed in subjects older than 20 years of age. Although nasal polyposis is not frequently observed in children under the age of 10, ciliary disorders, such as cystic fibrosis, come to mind when it is observed in this age group. There is a 2:1 male/female ratio in nasal polyposis. One in three patients with nasal polyps are also diagnosed with asthma, but only 7 per cent of asthma patients are diagnosed with this condition.Reference Settipane13
Viral factors, such as the Epstein–Barr virus (EBV), HPV and herpes simplex virus have been studied in nasal polyps. The existence of a high rate of EBV in patients with nasal polyps from Hong Kong has been shown using various techniques (15 per cent by Southern blot, 69 per cent by polymerase chain reaction and 85 per cent by in situ hybridisation. The EBV is very rare in nasal polyps whereas it is diagnosed in 88 per cent of patients with normal nasopharyngeal mucus; therefore, the contribution of the EBV to the aetiology of nasal polyps has not been taken into consideration among other possibilities.Reference Tao, Srivastava, Dickens and Ho14 In a study conducted in Canada in patients with nasal polyps, in situ hybridisation was used to identify EBV from DNA, but no EBV was identified.Reference Kozak, Mahony, Chernesky, Newhouse, Dolovich and Hitch15 Most probably, the difference observed in EBV positivity was due to the geographical differences of the study cases and the various techniques used in the studies. Polymerase chain reaction is believed to be a more sensitive technique than fluorescence in situ hybridisation, and we used the former technique to study virus expression in nasal mucosa.
Herpes simplex virus 1 occasionally causes oral and genital warts whereas herpes simplex virus 2 is the most frequent cause of genital herpes. In a study by Zaravinos et al., two positive nasal polyp samples (8 per cent) were diagnosed with polymerase chain reaction, but it was concluded that this was a chance result.Reference Zaravinos, Bizakis and Spandidos16
• The aetiology of nasal polyposis has been the subject of much research, but has not yet been established
• Viral, bacterial, fungal and protozoan agents have been implicated
• This study investigated the role of major respiratory viruses in the aetiology of nasal polyps using the polymerase chain reaction technique, the most sensitive method
• A significant role for adenovirus, Epstein-Barr virus, herpes simplex virus and human papilloma virus was not established
For the mucosal sites of the sinonasal cavity, a significant portion of inverted papillomas as well as squamous cell carcinomas have been shown to contain HPV DNA.Reference Shen, Tate, Crum and Goodman17 The absence of HPV DNA in nasal polyps supports the hypothesis of different pathological pathways leading to neoplastic and non-neoplastic mucosal lesions.Reference Becker, Forslund, Hansson and Malm18 Non-neoplastic mucosal lesions, such as inflammation-induced mucosal swellings in nasal polyposis, consist histopathologically of basement membrane thickening, atypical gland formation, goblet cell hyperplasia, inflammatory cell infiltration and subepithelial oedema.
The hypothesis of viral induction of proliferative mucosal lesions has been described.Reference Zur Hausen19 Based on this hypothesis, we investigated whether the major respiratory viruses affected the formation of nasal polyps by increasing proliferation on the nasal mucosa. Our results showed that there was no statistically significant relationship between the development of nasal polyposis and the major respiratory viruses (p < 0.001).
In conclusion, our results show that respiratory viruses are not responsible for inflammation-induced mucosal lesions in the nasal cavities. The genesis of nasal polyps needs to be investigated further, focusing on other causally related agents.