Published online by Cambridge University Press: 17 December 2019
Most network models describing solute transport in the brain microcirculation use the well-mixed hypothesis and assume that radial gradients inside the blood vessels are negligible. Recent experimental data suggest that these gradients, which may result from heterogeneities in the velocity field or consumption in the tissue, may in fact be important. Here, we study the validity of the well-mixed hypothesis in network models of solute transport using theoretical and computational approaches. We focus on regimes of weak coupling where the transport problem inside the vasculature is independent of the concentration field in the tissue. In these regimes, the boundary condition between vessels and tissue can be modelled by a Robin boundary condition. For this boundary condition and for a single cylindrical capillary, we derive a one-dimensional cross-section average transport problem with dispersion and exchange coefficients capturing the effects of radial gradients. We then extend this model to a network of connected tubes and solve the problem in a complex anatomical network. By comparing with results based on the well-mixed hypothesis, we find that dispersive effects are a fundamental component of transport in transient situations with relatively rapid injections, i.e. frequencies above one Hertz. For slowly varying signals and steady states, radial gradients also significantly impact the spatial distribution of vessel/tissue exchange for molecules that easily cross the blood brain barrier. This suggests that radial gradients cannot be systematically neglected and that there is a crucial need to determine the impact of spatio-temporal heterogeneities on transport in the brain microcirculation.
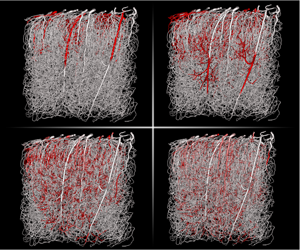
Transient numerical solution (1s) of average concentration of an intravascular tracer with a high diffusion coefficient ($D_V=10^{-9}m^2.s^{-1}$) resulting from a square input of 0.1s duration. This solution has been obtained using the WCA model on the network shown in figure 2a and using the velocity field computed in Cruz-Hernández et al. (2019).