1. Introduction
The fat mass and obesity associated (FTO) gene is associated with body mass index (BMI), waist circumference (WC) and obesity risk (Frayling et al., Reference Frayling, Timpson, Weedon, Zeggini, Freathy, Lindgren, Perry, Elliott, Lango, Rayner, Shields, Harries, Barrett, Ellard, Groves, Knight, Patch, Ness, Ebrahim, Lawlor, Ring, Ben-Shlomo, Jarvelin, Sovio, Bennett, Melzer, Ferrucci, Loos, Barroso, Wareham, Karpe, Owen, Cardon, Walker, Hitman, Palmer, Doney, Morris, Smith, Hattersley and McCarthy2007; Willer et al., Reference Willer, Speliotes, Loos, Li, Lindgren, Heid, Berndt, Elliott, Jackson, Lamina, Lettre, Lim, Lyon, McCarroll, Papadakis, Qi, Randall, Roccasecca, Sanna, Scheet, Weedon, Wheeler, Zhao, Jacobs, Prokopenko, Soranzo, Tanaka, Timpson, Almgren, Bennett, Bergman, Bingham, Bonnycastle, Brown, Burtt, Chines, Coin, Collins, Connell, Cooper, Smith, Dennison, Deodhar, Elliott, Erdos, Estrada, Evans, Gianniny, Gieger, Gillson, Guiducci, Hackett, Hadley, Hall, Havulinna, Hebebrand, Hofman, Isomaa, Jacobs, Johnson, Jousilahti, Jovanovic, Khaw, Kraft, Kuokkanen, Kuusisto, Laitinen, Lakatta, Luan, Luben, Mangino, McArdle, Meitinger, Mulas, Munroe, Narisu, Ness, Northstone, O'Rahilly, Purmann, Rees, Ridderstrale, Ring, Rivadeneira, Ruokonen, Sandhu, Saramies, Scott, Scuteri, Silander, Sims, Song, Stephens, Stevens, Stringham, Tung, Valle, Van Duijn, Vimaleswaran, Vollenweider, Waeber, Wallace, Watanabe, Waterworth, Watkins, Witteman, Zeggini, Zhai, Zillikens, Altshuler, Caulfield, Chanock, Farooqi, Ferrucci, Guralnik, Hattersley, Hu, Jarvelin, Laakso, Mooser, Ong, Ouwehand, Salomaa, Samani, Spector, Tuomi, Tuomilehto, Uda, Uitterlinden, Wareham, Deloukas, Frayling, Groop, Hayes, Hunter, Mohlke, Peltonen, Schlessinger, Strachan, Wichmann, McCarthy, Boehnke, Barroso, Abecasis and Hirschhorn2009), and has been implicated in food and total energy intakes (Cecil et al., Reference Cecil, Tavendale, Watt, Hetherington and Palmer2008; Speakman et al., Reference Speakman, Rance and Johnstone2008). These studies have been mainly in people of European origin but in the coming years, the greatest increases in obesity rates will occur in Asian countries, therefore, investigation of genetic associations in these populations is warranted. Genome-wide association studies have identified at least 50 genetic loci that are associated with obesity-related traits. The locus that was first identified as an obesity-susceptibility locus is the fat mass and obesity associated (FTO) gene in which genetic variation (e.g. the rs993609 SNP) has been convincingly associated with BMI, WC and obesity risk (Frayling et al., Reference Frayling, Timpson, Weedon, Zeggini, Freathy, Lindgren, Perry, Elliott, Lango, Rayner, Shields, Harries, Barrett, Ellard, Groves, Knight, Patch, Ness, Ebrahim, Lawlor, Ring, Ben-Shlomo, Jarvelin, Sovio, Bennett, Melzer, Ferrucci, Loos, Barroso, Wareham, Karpe, Owen, Cardon, Walker, Hitman, Palmer, Doney, Morris, Smith, Hattersley and McCarthy2007; Andreasen et al., Reference Andreasen, Stender-Petersen, Mogensen, Torekov, Wegner, Andersen, Nielsen, Albrechtsen, Borch-Johnsen, Rasmussen, Clausen, Sandbaek, Lauritzen, Hansen, Jorgensen, Pedersen and Hansen2008; Bauer et al., Reference Bauer, Elbers, Adan, Loos, Onland-Moret, Grobbee, van Vliet-Ostaptchouk, Wijmenga and van der Schouw2009; Willer et al., Reference Willer, Speliotes, Loos, Li, Lindgren, Heid, Berndt, Elliott, Jackson, Lamina, Lettre, Lim, Lyon, McCarroll, Papadakis, Qi, Randall, Roccasecca, Sanna, Scheet, Weedon, Wheeler, Zhao, Jacobs, Prokopenko, Soranzo, Tanaka, Timpson, Almgren, Bennett, Bergman, Bingham, Bonnycastle, Brown, Burtt, Chines, Coin, Collins, Connell, Cooper, Smith, Dennison, Deodhar, Elliott, Erdos, Estrada, Evans, Gianniny, Gieger, Gillson, Guiducci, Hackett, Hadley, Hall, Havulinna, Hebebrand, Hofman, Isomaa, Jacobs, Johnson, Jousilahti, Jovanovic, Khaw, Kraft, Kuokkanen, Kuusisto, Laitinen, Lakatta, Luan, Luben, Mangino, McArdle, Meitinger, Mulas, Munroe, Narisu, Ness, Northstone, O'Rahilly, Purmann, Rees, Ridderstrale, Ring, Rivadeneira, Ruokonen, Sandhu, Saramies, Scott, Scuteri, Silander, Sims, Song, Stephens, Stevens, Stringham, Tung, Valle, Van Duijn, Vimaleswaran, Vollenweider, Waeber, Wallace, Watanabe, Waterworth, Watkins, Witteman, Zeggini, Zhai, Zillikens, Altshuler, Caulfield, Chanock, Farooqi, Ferrucci, Guralnik, Hattersley, Hu, Jarvelin, Laakso, Mooser, Ong, Ouwehand, Salomaa, Samani, Spector, Tuomi, Tuomilehto, Uda, Uitterlinden, Wareham, Deloukas, Frayling, Groop, Hayes, Hunter, Mohlke, Peltonen, Schlessinger, Strachan, Wichmann, McCarthy, Boehnke, Barroso, Abecasis and Hirschhorn2009). To date, only a handful of studies have investigated associations between the rs9939609 variant with obesity in Asian populations. These studies reported the rs9939609 variant to be associated with increased BMI and/or WC in Chinese (Chang et al., Reference Chang, Liu, Lee, Chang, Jiang, Li, Kuo, Lee and Chuang2008; Li et al., Reference Li, Song, Jiang, Zhang, Lin, Bao, Yao, Yang, Hao and Liu2010) and South Asian people (Al-Attar et al., Reference Al-Attar, Pollex, Ban, Young, Bjerregaard, Anand, Yusuf, Zinman, Harris, Hanley, Connelly, Huff and Hegele2008). Furthermore, this association has also been reported in North American Aboriginals (Al-Attar et al., Reference Al-Attar, Pollex, Ban, Young, Bjerregaard, Anand, Yusuf, Zinman, Harris, Hanley, Connelly, Huff and Hegele2008; Rong et al., Reference Rong, Hanson, Ortiz, Wiedrich, Kobes, Knowler, Bogardus and Baier2009). However, not all of these studies are consistent as several others have found no associations in these populations, and none was conducted in multiple ethnic groups living in the same environment (Al-Attar et al., Reference Al-Attar, Pollex, Ban, Young, Bjerregaard, Anand, Yusuf, Zinman, Harris, Hanley, Connelly, Huff and Hegele2008; Li et al., Reference Li, Wu, Loos, Hu, Liu, Wang, Yu and Lin2008; Rong et al., Reference Rong, Hanson, Ortiz, Wiedrich, Kobes, Knowler, Bogardus and Baier2009; Yajnik et al., Reference Yajnik, Janipalli, Bhaskar, Kulkarni, Freathy, Prakash, Mani, Weedon, Kale, Deshpande, Krishnaveni, Veena, Fall, McCarthy, Frayling, Hattersley and Chandak2009).
Many of the above studies have focused on anthropometric measures, but there is general agreement that BMI does not distinguish between individuals of increased weight due to excess body fat or greater muscle mass. The WC is limited in its ability to reflect total body fat and is unable to discriminate between the more metabolically active inner abdominal adipose tissue (visceral adipose tissue (VAT)) and subcutaneous abdominal adipose tissue (SAT). Our earlier research has indicated that Chinese and South Asians have a unique phenotype of increased VAT compared with Europeans of the same body size (Lear et al., Reference Lear, Humphries, Kohli and Birmingham2007a, Reference Lear, Humphries, Kohli, Chockalingam and Frohlicha). Therefore, the purpose of this investigation was to assess the association of the FTO rs9939609 variant with measures of body fat using precise imaging techniques in a multi-ethnic adult population and report on the effect sizes. We further assessed the association of the FTO variants with total and macronutrient energy intakes because studies have shown associations between homozygosity for the rs9939609 minor allele with food and total energy intakes in children (Cecil et al., Reference Cecil, Tavendale, Watt, Hetherington and Palmer2008; Speakman et al., Reference Speakman, Rance and Johnstone2008; Wardle et al., Reference Wardle, Carnell, Haworth, Farooqi, O'Rahilly and Plomin2008, Reference Wardle, Llewellyn, Sanderson and Plomin2009).
2. Materials and methods
Participants for this study were from the Multi-cultural Community Health Assessment Trial (M-CHAT) (Lear et al., Reference Lear, Birmingham, Chockalingam and Humphries2006). Apparently healthy men and women between 30 and 65 years of age were recruited from the greater Vancouver area in Canada of either Aboriginal (reserve and non-reserve residents), Chinese (China, Hong Kong and Taiwan), European (continental Europe, Ireland and UK) or South Asian (Bangladesh, India, Nepal, Pakistan and Sri Lanka) origin. Based on self-report, participants must have indicated that all known ancestors were either of Aboriginal origin or descended from one of the three geographical areas. Due to the high prevalence of mixed ethnic origins in Aboriginal populations, Aboriginals with at least three grandparents of exclusive Aboriginal origin were recruited into the main M-CHAT study; however, only those who reported that all four grandparents were of exclusive Aboriginal origin were included in the present analyses. Of the 828 M-CHAT participants recruited, 742 provided consent for DNA collection, after excluding Aboriginal participants without four Aboriginal grandparents, the sample size available for analysis was 706 (131 Aboriginals, 202 Chinese, 184 Europeans and 189 South Asians). The study was approved by the Simon Fraser University and Children and Family Research Institute Research Ethics Boards.
(i) Participant assessment
Participants were assessed for diet, anthropometry, intra-abdominal fat, total body fat and provided a blood sample for DNA extraction. All data were collected on the same day, except for the blood sample that was collected within 3 weeks of the main assessment.
(ii) Body composition assessment
Weight was assessed using a balance-beam scale with participants in light street clothing, footwear removed and pockets emptied. Height was assessed by stadiometer and BMI was calculated as weight in kilograms divided by height in metres squared. Waist circumference was recorded in centimetres as the average of two measures taken against the skin at maximal narrowing of the waist following a normal expiration. Hip circumference was recorded in centimetres as the average of two measures taken at the point of maximal gluteal protuberance from the lateral view over undergarments.
Inner abdominal fat was assessed by computer tomography (CT) scan using a CTi Advantage scanner (General Electric, Milwaukee, WI). A cross-sectional 10 mm slice at the L4/L5 intrevertebral disc was obtained and the attenuation range of −190 to −30 Hounsfield units was used to identify adipose tissue. Computation of surface areas from the CT scans was conducted using SliceOmatic 4.2 medical imaging software (SliceOmatic v.4.2, Tomovision, Montreal). Total abdominal fat was calculated as all pixels within the attenuation range and VAT was defined as adipose tissue within the inside edge of the abdominal wall. SAT was the difference between total abdominal adipose tissue and VAT.
Total body fat was assessed by dual energy X-ray absorptiometry with a Norland XR-36 scanner (Norland Medical Systems, White Plains, NY) using Host Software Version 3.9.4 and Scanner Software 2.1.0. Where possible, participants removed all jewellery and metallic objects that could potentially affect the scan results. In some instances, participants could not remove rings from fingers or bracelets from their wrist. Per cent total body fat was calculated as total body fat divided by total body mass. Peripheral fat mass was the sum of fat mass in the arms and legs. Per cent peripheral fat mass was calculated as peripheral fat mass divided by total body mass.
(iii) DNA extraction and genotyping
DNA was extracted from whole blood samples using the QIAamp DNA Blood kit (Qiagen) following the manufacturer's suggested protocol. Genotyping of the FTO rs9939609 variant was performed by real-time PCR using TaqMan pre-designed SNP genotyping assays (Applied Biosystems) and a 7500 Real-Time PCR System (Applied Biosystems with a call rate of 100%). Table 1 presents data on the minor allele and genotype frequencies. The rs9939609 variant was in Hardy–Weinberg equilibrium in each ethnic group (P>0·05).
Table 1. Participant demographics, allele and genotype frequencies, dietary and anthropometric data
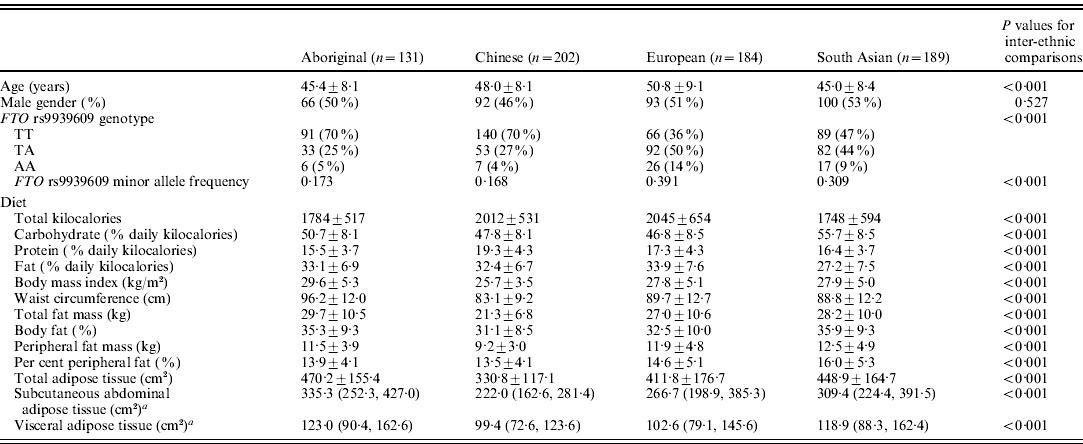
a Medians and 25th and 75th percentile values presented.
Data are expressed as means and sd unless otherwise indicated.
(iv) Statistical analyses
Continuous variables are presented as means±sd, and categorical variables as percentages and counts. Visceral adipose tissue and SAT had non-normal distributions and were log transformed prior to analyses. Within each ethnic group additive linear regression was used to identify the association of the rs9939609 genotype with the anthropometric, body fat and dietary data adjusted for age and sex. As the associations between the FTO genotype and per cent daily calories from carbohydrates were in opposite directions for the Aboriginals and Europeans, we also tested for an inter-ethnic interaction for the dietary outcomes. There were 34 participants with incomplete dietary records, resulting in 672 participants included in the regression models with the dietary outcomes. To ensure that insignificant P values were not due to the low sample sizes within each ethnic group, we conducted an analysis combining all ethnic groups using the same linear models previously described and adding three indicator variables to account for inter-ethnic differences. Based on an effect size for the rs9939609 minor allele of 0·34% of the variance in BMI (Willer et al., Reference Willer, Speliotes, Loos, Li, Lindgren, Heid, Berndt, Elliott, Jackson, Lamina, Lettre, Lim, Lyon, McCarroll, Papadakis, Qi, Randall, Roccasecca, Sanna, Scheet, Weedon, Wheeler, Zhao, Jacobs, Prokopenko, Soranzo, Tanaka, Timpson, Almgren, Bennett, Bergman, Bingham, Bonnycastle, Brown, Burtt, Chines, Coin, Collins, Connell, Cooper, Smith, Dennison, Deodhar, Elliott, Erdos, Estrada, Evans, Gianniny, Gieger, Gillson, Guiducci, Hackett, Hadley, Hall, Havulinna, Hebebrand, Hofman, Isomaa, Jacobs, Johnson, Jousilahti, Jovanovic, Khaw, Kraft, Kuokkanen, Kuusisto, Laitinen, Lakatta, Luan, Luben, Mangino, McArdle, Meitinger, Mulas, Munroe, Narisu, Ness, Northstone, O'Rahilly, Purmann, Rees, Ridderstrale, Ring, Rivadeneira, Ruokonen, Sandhu, Saramies, Scott, Scuteri, Silander, Sims, Song, Stephens, Stevens, Stringham, Tung, Valle, Van Duijn, Vimaleswaran, Vollenweider, Waeber, Wallace, Watanabe, Waterworth, Watkins, Witteman, Zeggini, Zhai, Zillikens, Altshuler, Caulfield, Chanock, Farooqi, Ferrucci, Guralnik, Hattersley, Hu, Jarvelin, Laakso, Mooser, Ong, Ouwehand, Salomaa, Samani, Spector, Tuomi, Tuomilehto, Uda, Uitterlinden, Wareham, Deloukas, Frayling, Groop, Hayes, Hunter, Mohlke, Peltonen, Schlessinger, Strachan, Wichmann, McCarthy, Boehnke, Barroso, Abecasis and Hirschhorn2009) and an alpha of 0·1 (one-sided), our power to detect an association is 46·3% power (for a power of 80%, a sample of 1813 participants is need). All analyses were conducted using R and the α-level was set at <0·05 (one-sided) for significance.
3. Results
Table 1 outlines the minor allele and genotype frequencies, and the outcome variables. The minor allele and genotype frequencies differed among the ethnic groups (P<0·001). Europeans and South Asians had greater minor allele frequencies compared with the Aboriginals and Chinese (P<0·001 for all comparisons). There were no differences between the Europeans and South Asians, or the Aboriginals and Chinese. Dietary and anthropometric measures were significantly different across the four ethnic groups (P<0·001 for all).
In Aboriginals, the rs9939609 minor allele was associated with a −2·2±1·3 and 1·1±0·6% absolute change in per cent daily calories from carbohydrate (P=0·049) and proteins (P=0·029), respectively (Table 2). In Europeans, the rs9939609 minor allele was associated with a 9·7±5·7% relative increase in VAT (P=0·047) and a 2·3±0·9% absolute increase in percent daily calories from carbohydrates (P=0·007). There was a significant inter-ethnic interaction between ethnicity (Aboriginal versus European) and the FTO genotype for per cent daily calories from carbohydrates (P=0·004), there were no other significant interactions for dietary outcomes (data not shown). There were no other significant associations within the ethnic groups. When the four ethnic groups were analysed together (Table 2), there were significant associations between the rs9939609 minor allele with greater fat mass (0·94±0·56 kg, P=0·045), per cent body fat (0·7±0·4%, P=0·031) and relative greater SAT body fat (4·9±2·8%, P=0·039).
Table 2. Beta coefficients of the FTO rs9939609 minor allele for the dependent body composition and food intake variables
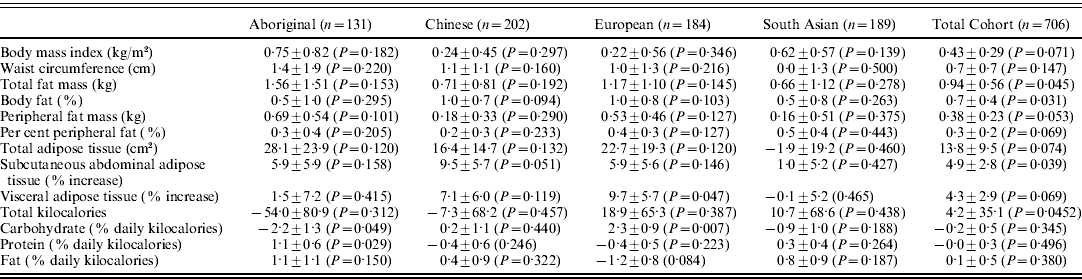
Adjusted for age and sex. Data are reported as per the allele change and sd with P values in parentheses.
4. Discussion
The purpose of this study was to assess the association and report effect sizes between the rs9939609 SNP in the FTO gene with direct measures of body fat using imaging techniques in a multi-ethnic cohort. We found differences in minor allele frequency among the four groups such that the frequency of the minor allele was highest in the European and South Asian groups, and lowest in Aboriginal and Chinese groups. In Aboriginals and Europeans, the minor allele was associated with dietary intake and VAT (Europeans only). When the four ethnic groups were analysed together, the FTO rs9939609 minor allele was also associated with body fat measures and SAT.
The minor allele frequencies reported in the present study are similar to that found in a number of studies investigating similar ethnic groups (Al-Attar et al., Reference Al-Attar, Pollex, Ban, Young, Bjerregaard, Anand, Yusuf, Zinman, Harris, Hanley, Connelly, Huff and Hegele2008; Li et al., Reference Li, Wu, Loos, Hu, Liu, Wang, Yu and Lin2008; Sanghera et al., Reference Sanghera, Ortega, Han, Singh, Ralhan, Wander, Mehra, Mulvihill, Ferrell, Nath and Kamboh2008; Rong et al., Reference Rong, Hanson, Ortiz, Wiedrich, Kobes, Knowler, Bogardus and Baier2009; Yajnik et al., Reference Yajnik, Janipalli, Bhaskar, Kulkarni, Freathy, Prakash, Mani, Weedon, Kale, Deshpande, Krishnaveni, Veena, Fall, McCarthy, Frayling, Hattersley and Chandak2009). Our investigation adds to this by providing direct comparisons of allele frequency in these ethnic groups recruited under the same criteria and indicates that the minor allele frequency is no different in Europeans and South Asians, and higher in Aboriginals and Chinese, with no difference between these two latter groups.
Although not significant, our per minor allele effect size on BMI was highest in Aboriginals (0·75 kg/m2) and similar to that reported by Rong et al. (0·8 kg/m2) (Rong et al., Reference Rong, Hanson, Ortiz, Wiedrich, Kobes, Knowler, Bogardus and Baier2009). Studies in Chinese populations have reported the FTO rs9939609 variant to be associated with increased obesity (Li et al., Reference Li, Song, Jiang, Zhang, Lin, Bao, Yao, Yang, Hao and Liu2010) with the effect sizes of the minor allele for BMI ranging from 0·37 to 0·68 kg/m2 (Chang et al., Reference Chang, Liu, Lee, Chang, Jiang, Li, Kuo, Lee and Chuang2008; Tan et al., Reference Tan, Dorajoo, Seielstad, Sim, Ong, Chia, Wong, Saw, Chew, Aung and Tai2008), which is somewhat higher than what we reported. However, not all studies are consistent as Li et al. did not report an association between the minor allele and BMI in 3210 Chinese men and women (Li et al., Reference Li, Wu, Loos, Hu, Liu, Wang, Yu and Lin2008). For Europeans, our per minor allele effect size for BMI was 0·22 kg/m2 and similar to that reported in larger studies of Europeans (Frayling et al., Reference Frayling, Timpson, Weedon, Zeggini, Freathy, Lindgren, Perry, Elliott, Lango, Rayner, Shields, Harries, Barrett, Ellard, Groves, Knight, Patch, Ness, Ebrahim, Lawlor, Ring, Ben-Shlomo, Jarvelin, Sovio, Bennett, Melzer, Ferrucci, Loos, Barroso, Wareham, Karpe, Owen, Cardon, Walker, Hitman, Palmer, Doney, Morris, Smith, Hattersley and McCarthy2007; Willer et al., Reference Willer, Speliotes, Loos, Li, Lindgren, Heid, Berndt, Elliott, Jackson, Lamina, Lettre, Lim, Lyon, McCarroll, Papadakis, Qi, Randall, Roccasecca, Sanna, Scheet, Weedon, Wheeler, Zhao, Jacobs, Prokopenko, Soranzo, Tanaka, Timpson, Almgren, Bennett, Bergman, Bingham, Bonnycastle, Brown, Burtt, Chines, Coin, Collins, Connell, Cooper, Smith, Dennison, Deodhar, Elliott, Erdos, Estrada, Evans, Gianniny, Gieger, Gillson, Guiducci, Hackett, Hadley, Hall, Havulinna, Hebebrand, Hofman, Isomaa, Jacobs, Johnson, Jousilahti, Jovanovic, Khaw, Kraft, Kuokkanen, Kuusisto, Laitinen, Lakatta, Luan, Luben, Mangino, McArdle, Meitinger, Mulas, Munroe, Narisu, Ness, Northstone, O'Rahilly, Purmann, Rees, Ridderstrale, Ring, Rivadeneira, Ruokonen, Sandhu, Saramies, Scott, Scuteri, Silander, Sims, Song, Stephens, Stevens, Stringham, Tung, Valle, Van Duijn, Vimaleswaran, Vollenweider, Waeber, Wallace, Watanabe, Waterworth, Watkins, Witteman, Zeggini, Zhai, Zillikens, Altshuler, Caulfield, Chanock, Farooqi, Ferrucci, Guralnik, Hattersley, Hu, Jarvelin, Laakso, Mooser, Ong, Ouwehand, Salomaa, Samani, Spector, Tuomi, Tuomilehto, Uda, Uitterlinden, Wareham, Deloukas, Frayling, Groop, Hayes, Hunter, Mohlke, Peltonen, Schlessinger, Strachan, Wichmann, McCarthy, Boehnke, Barroso, Abecasis and Hirschhorn2009). In South Asians, while this variant is associated with risk for type 2 diabetes (Sanghera et al., Reference Sanghera, Ortega, Han, Singh, Ralhan, Wander, Mehra, Mulvihill, Ferrell, Nath and Kamboh2008; Yajnik et al., Reference Yajnik, Janipalli, Bhaskar, Kulkarni, Freathy, Prakash, Mani, Weedon, Kale, Deshpande, Krishnaveni, Veena, Fall, McCarthy, Frayling, Hattersley and Chandak2009), no associations with BMI have been observed in these and other studies (Al-Attar et al., Reference Al-Attar, Pollex, Ban, Young, Bjerregaard, Anand, Yusuf, Zinman, Harris, Hanley, Connelly, Huff and Hegele2008; Tan et al., Reference Tan, Dorajoo, Seielstad, Sim, Ong, Chia, Wong, Saw, Chew, Aung and Tai2008). One study did report a non-significant effect size on BMI of 0·1 kg/m2 (Tan et al., Reference Tan, Dorajoo, Seielstad, Sim, Ong, Chia, Wong, Saw, Chew, Aung and Tai2008), which is less than the 0·6 kg/m2 we report. Although not significant, our reported effect sizes per minor allele for BMI are similar to that of previous studies apart from that in South Asians in which we report a much higher effect.
We found positive associations between the FTO rs9939609 variant with body fat mass, Per cent body fat and SAT when the groups were analysed together. Previous studies have found the FTO rs8050136 minor allele to be associated with a 1·9% and 2·5 kg increase in body fat as assessed by bioelectrical impedance and an increase in SAT volume in Europeans (Haupt et al., Reference Haupt, Thamer, Machann, Kirchhoff, Stefan, Tschritter, Machicao, Schick, Haring and Fritsche2008). The more than twofold higher effect sizes reported in this study compared with ours may be the result of the use of bioelectrical impedance compared with the use of DEXA scanning. In another study, the rs1558902 and rs1421085 FTO minor alleles were found to be associated with approximately 14·5 cm2 (6·5%) more SAT and 5·8 cm2 (4·7%) more VAT in Japanese men and women (Hotta et al., Reference Hotta, Nakamura, Nakamura, Matsuo, Nakata, Kamohara, Miyatake, Kotani, Komatsu, Itoh, Mineo, Wada, Yoneda, Nakajima, Funahashi, Miyazaki, Tokunaga, Kawamoto, Masuzaki, Ueno, Hamaguchi, Tanaka, Yamada, Hanafusa, Oikawa, Yoshimatsu, Nakao, Sakata, Matsuzawa, Nakamura and Kamatani2010). These effect sizes are consistent with our findings of 4·9 and 4·3%, respectively. Together, these results suggest that FTO may have a role in body fat distribution. Indeed, FTO mRNA expression has been shown to be three times higher in SAT than in VAT from 55 lean and obese men (Kloting et al., Reference Kloting, Schleinitz, Ruschke, Berndt, Fasshauer, Tonjes, Schon, Kovacs, Stumvoll and Bluher2008).
We observed an inter-ethnic interaction between Aboriginals and Europeans with respect to the association of the FTO rs9939609 variant with per cent daily calories from carbohydrate such that in Aboriginals, the FTO rs9939609 variant to be negatively associated with dietary caloric intake from carbohydrates, while the FTO rs9939609 variant was positively associated with dietary caloric intake from carbohydrates. It is unclear what the mechanism is for the inter-ethnic differences in dietary intake, but this may be due to the differences in the types of carbohydrate in their diets. We cannot exclude the possibility that there may be inter-ethnic differences in the reporting of dietary intake on the three-day food record. Previous studies have suggested that FTO is involved in energy homoeostasis and food preferences (Cecil et al., Reference Cecil, Tavendale, Watt, Hetherington and Palmer2008; Tanofsky-Kraff et al., Reference Tanofsky-Kraff, Han, Anandalingam, Shomaker, Columbo, Wolkoff, Kozlosky, Elliott, Ranzenhofer, Roza, Yanovski and Yanovski2009), while others have not found this association (Bauer et al., Reference Bauer, Elbers, Adan, Loos, Onland-Moret, Grobbee, van Vliet-Ostaptchouk, Wijmenga and van der Schouw2009; Hakanen et al., Reference Hakanen, Raitakari, Lehtimaki, Peltonen, Pahkala, Sillanmaki, Lagstrom, Viikari, Simell and Ronnemaa2009; Liu et al., Reference Liu, Zhu, Lagou, Gutin, Stallmann-Jorgensen, Treiber, Dong and Snieder2010). Interestingly all but one of these studies was in children; the lone adult one found no association between a different FTO variant (rs1121980) and nutrient intake in women (Bauer et al., Reference Bauer, Elbers, Adan, Loos, Onland-Moret, Grobbee, van Vliet-Ostaptchouk, Wijmenga and van der Schouw2009). At present, it is unclear why our findings are not consistent with the other study in adults, but it may be due to differences in the variants, our assessment of both men and women, and/or the assessment of dietary intake (food frequency questionnaire compared with our three-day food record). As a result, it remains unknown whether the associations reported in children do indeed extend to adults. However, studies in mice indicate that FTO mRNA is highly expressed in hypothalamus and regulated by fasting and refeeding (Gerken et al., Reference Gerken, Girard, Tung, Webby, Saudek, Hewitson, Yeo, McDonough, Cunliffe, McNeill, Galvanovskis, Rorsman, Robins, Prieur, Coll, Ma, Jovanovic, Farooqi, Sedgwick, Barroso, Lindahl, Ponting, Ashcroft, O'Rahilly and Schofield2007) and while the biological function of FTO has not been fully characterized, mice overexpressing one or two additional copies of FTO have a dose-dependent increase in body and fat mass and increased food intake (Church et al., Reference Church, Moir, McMurray, Girard, Banks, Teboul, Wells, Bruning, Nolan, Ashcroft and Cox2010) supporting a role for FTO in food intake and energy homoeostasis.
(i) Limitations
The small sample size of our study may have limited the ability to detect certain associations, particularly within ethnic groups. However, our effect sizes for BMI are similar to that reported in previous studies giving us confidence that our analyses of associations with discrete areas of body composition using accurate imaging techniques provide novel information on effect sizes for these outcomes. We must also acknowledge the limitation of multiple comparisons; however, the majority of literature to date would indicate that the FTO variant is associated with measures of body fat and food intake. As our findings are consistent with the previous literature, we believe the likelihood of spurious findings is low. Lastly, as our study is cross-sectional, we are limited to identifying associations only and longitudinal studies investigating changes in body fat over time are needed to identify and understand more clearly the role of FTO.
(ii) Conclusions
A unique contribution of our investigation is the comparison of precise body fat imaging and dietary measures in a cohort of different ethnic groups recruited from the same environment. Our results confirm that the frequency of the minor allele of the FTO rs9939609 variant differs across ethnic groups and the presence of minor allele may be associated with increased body fat and SAT independent of ethnicity. In addition, our finding that the FTO rs9939609 variant is associated with energy intake supports a possible role for FTO in relation to obesity that has been observed in studies of children. Although our study is limited by a small sample size, our reported per allele effect sizes for BMI are consistent with previous studies. These results suggest that carriers of the minor rs9939609 allele may be at increased risk for excess body fat, and therefore at greater risk for cardiometabolic diseases.
This research was funded by the Canadian Institutes of Health Research. Dr Lear holds the Pfizer/Heart & Stroke Foundation Chair in Cardiovascular Prevention Research at St. Paul's Hospital and is a Canadian Institutes of Health Research New Investigator. Dr Devlin is a New Investigator of the Heart and Stroke Foundation of Canada. The authors had complete independence from the funders.
5. Declaration of Interest
None.




