No CrossRef data available.
Published online by Cambridge University Press: 16 April 2020
Rapidly dissolving oral dispersible tablets (ODT) have been developed to overcome problems related to swallowing.
Establish bioequivalence between ODT and the immediate release (IR) escitalopram tablet and determine its perception by healthy subjects.
In a randomized, open-label, cross-over design, 30 healthy men received 20 mg escitalopram as ODT tablets (2 × 10 mg or 1 × 20 mg) or conventional tablets. Twenty blood samples were collected after each dose administration and pharmacokinetic parameters were determined using non-compartmental methods. Safety was assessed by self-reported adverse events (AE) and vital signs. Subjects completed a questionnaire relating to their perception of the ODT.
Statistical analysis of systemic exposure to escitalopram showed that ODT was bioequivalent to IR escitalopram for the primary (log-transformed AUC0-inf and Cmax) and secondary parameters (Table 1). AE incidence was similar for both dosage forms and all AEs considered related to escitalopram were mild. There were no serious AEs. Subjects found the ODT to have a pleasant texture (98%), size (95%), a pleasant mint/peppermint taste (86%), and suitable for long-term treatment (96%).
ODT escitalopram was bioequivalent to the conventional tablet. Based on the subjects’ perception of taste, texture and size ODT escitalopram is a convenient and pleasant alternative to the conventional tablet.
[Table 1]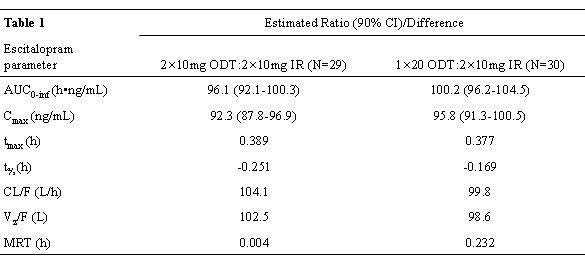
Comments
No Comments have been published for this article.