Mycobacterium avium subsp. avium (M. a. avium) is the causal agent of avian tuberculosis especially in domestic and wild birds [Reference Shitaye1, Reference Moravkova2]. Infected birds shed large numbers of the organism into the environment, where it keeps its virulence and represents a source of infection for other animals and humans [Reference Shitaye1]. Although at a low incidence, M. a. avium usually causes pulmonary disease [Reference Marras and Daley3], but it can also infect the small intestine [Reference Sun4]. This is a frequent complication in AIDS patients, leading to intolerance to antiretroviral therapy or general worsening of the condition. M. a. avium infection is considered to be acquired from environmental sources, most commonly water [Reference Falkinham5]. M. neoaurum has rarely been found in humans, and there are only a small number of reports of M. neoaurum infection in immunocompromised patients. Infection has usually been associated with catheter placement or generalized mycobacteraemia; M. neoaurum has mostly been found in the blood of patients [Reference Brown-Elliott6]. A case of pulmonary infection in a woman with a history of asthma has also been reported [Reference Morimoto7]. The hospital environment is thought to be a common feature of almost all of the described infections, with water identified as the source of infection in one study [Reference Baird8].
We describe the case of a 17-year-old male patient. The patient's primary diagnosis was Burkitt's lymphoma in the neck area, confirmed by translocation t8;14 (q24,q32). He was successfully treated according to the Berlin-Frankfurt-Munich (BFM) 95 protocol for treatment of oncology patients. Four years later he developed mediastinal T-cell non-Hodgkin's lymphoma (T-NHL) as a secondary malignancy. During a period of almost 5 years of anti-cancer treatment he had been repeatedly admitted to hospital, as over 80% of the chemotherapy cycles were followed by episodes of neutropenic fever. During the late intensification phase of his second lymphoma therapy he developed febrile neutropenia with massive pleural effusion, requiring drainage. X-rays and CT examinations revealed bronchopneumonia on the left lung and bilateral sinusitis. He did not respond to standard empirical treatment with antibiotics and antifungal agents. Clinical improvement was very slow after prolonged treatment with cotrimoxazole. The cause was not established until the sputum samples were examined for the presence of mycobacteria.
During the hospitalization and anti-cancer treatment he suffered from diarrhoea and his stool bore mucosal fragments; granulomatous inflammation was diagnosed by standard histological examination. These fragments were negative for the presence of fungal pathogens.
Mycobacterial cultures of the sputum samples repeatedly yielded M. neoaurum isolations. The mycobacterial cultures of the fragments of intestinal mucosa were negative; however, the presence of M. a. avium was confirmed by IS901 qPCR, performed according to Slana et al. [Reference Slana9].
The patient received ciprofloxacin treatment for 3 months, and his condition improved markedly. He was on continuous complete remission of his lymphomas for two more years, when he developed tertiary malignancy, acute myeloid leukaemia, to which he eventually succumbed, 7 years after initial diagnosis and 3 years after the mycobacterial infection.
Thirty-two samples from the oncology unit in the university hospital were collected in order to establish possible sources of mycobacteria. The hospital is a large, well-equipped NIAHO (National Integrated Accreditation for Healthcare Organizations) accredited tertiary referral centre where standard disinfection and maintenance regimens apply. In the ‘closed unit’ used for patients on chemotherapy, air circulation is controlled by differential pressures and equipped with HEPA filters. The patient was living in a rural area, without any animals in his home, although animals were kept in most of the surrounding households. From the patient's house and garden 76 environmental samples were collected (samples consisted of garden soil, roots and leaves of vegetables and herbs grown in the garden, as well as spider webs, dust, water and sediment) including nine environmental samples from the neighbour's chicken coop where a small flock of hens supplied the patient's family with eggs (Table 1). The neighbour's residence was a few metres away and the patient sometimes helped to collect the eggs. The environmental samples were examined by culture as described previously [Reference Shitaye1]. Isolates were identified by sequencing of the 16S rRNA gene according to Harmsen et al. [Reference Harmsen10]. Nine environmental samples originating from the flock of hens belonging to the neighbour were additionally tested by IS901 qPCR [Reference Slana9]. From the environmental samples from the hospital and patient's house, 37 isolates were recovered. The species identified included M. gordonae, M. intracellulare, M. interjectum, M. engbaekii, M. a. hominissuis, M. kumamotonense, and M. celatum. There was no M. neoaurum isolate in the samples collected from the environment, and thus the source of M. neoaurum infection was not established. M. a. avium was detected by qPCR in environmental samples from the neighbour's chicken coop. Many species of environmental mycobacteria were found in the home and close surroundings of the patient.
Table 1. Examination of the patient's environment in the hospital and his home by mycobacterial culture
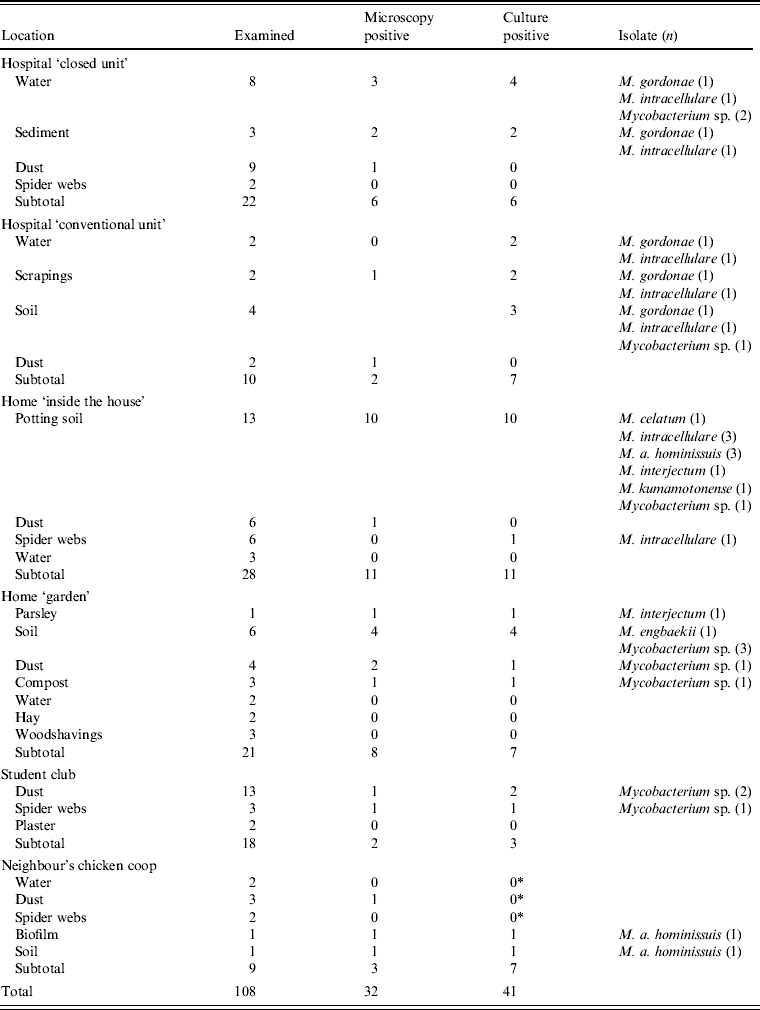
* M. a. avium was detected by qPCR according to Slana et al. [Reference Slana9] in amounts of 102–105 cells/g.
In the case of intestinal infection with M. a. avium, contaminated hen eggs are the probable source of infection. Although a link between the M. a. avium found in the patient and that found in the hens' environment has not been proven due to the lack of isolates, repeated exposure to high amounts of the infectious agent could contribute to infection/colonization.
In the developed world, there is a steady increase in people suffering from impairments of the immune system, with increased susceptibility to mycobacterial infections. Thus, it is important to increase public awareness of the possible routes of infection with environmental mycobacteria, especially since there are now more ecological farms where the animals are at higher risk of infections. Consumption of food originating from infected animals, or from animals living in environments harbouring high numbers of mycobacteria should be considered risky for immunocompromised individuals.
ACKNOWLEDGEMENTS
The work was supported by grants from the Ministry of Agriculture (No. MZe0002716202) and the Ministry of Education, Youth and Sports (AdmireVet, CZ 1.05/2.1.00/01.0006; ED0006/01/01.) of the Czech Republic.
DECLARATION OF INTEREST
None.



