Introduction
Haemolytic uraemic syndrome (HUS) is a relatively rare but severe multisystem syndrome clinically characterised by a triad of clinical markers: acute kidney injury (AKI), microangiopathic haemolytic anaemia (MAHA), and thrombocytopenia. Incomplete or partial forms of HUS (iHUS) have also been described in which the condition occurs with the absence of either MAHA or thrombocytopenia [Reference Bell, Griffin, Lozano, Christie, Kobayashi and Tarr1–Reference Sallée, Ismail, Fakhouri, Vacher-Coponat, Moussi-Francés, Frémaux-Bacchi and Burtey4].
The classification of HUS is contentious and constantly evolving. Historically, HUS was categorised by diarrhoeal prodrome, as D+ (diarrhoeal) and D- HUS (non-diarrhoeal), considered equivalent to typical HUS (tHUS) and atypical HUS (aHUS), respectively. Typical HUS is most often caused by infection with Shiga toxin-producing Escherichia coli (STEC-HUS, also referred to as eHUS), although other enteric pathogens including Shigella dysenteriae, Shigella sonnei, Salmonella, and Campylobacter species have also been isolated from stools of cases of tHUS [Reference Proulx and Sockett5–Reference Houdouin, Doit, Mariani, Brahimi, Loirat, Bourrillon and Bingen7]. While most tHUS cases experience a diarrhoeal prodrome, often with bloody stools, some do not experience diarrhoeal illness [Reference Tarr, Gordon and Chandler8]. Rarely, STEC causes tHUS in people without a diarrhoeal history but with symptoms of extra-intestinal infection, most often urinary tract infections [Reference Starr, Bennett-Wood, Bigham, de Koning-Ward, Bordun, Lightfoot, Bettelheim, Jones and Robins-Browne9–Reference Elliott, Robins-Browne, O’Loughlin, Bennett-Wood, Bourke, Henning, Hogg, Knight, Powell and Redmond12].
Infection with Streptococcus pneumoniae represents another major infectious cause of HUS with a separate pathogenic pathway to STEC and should be considered as a separate class of HUS, pneumococcal HUS (pHUS) [Reference Schifferli, von, Fontana, Spartà, Schmid, Bianchetti and Rudin11–Reference Loirat, Fakhouri, Ariceta, Besbas, Bitzan, Bjerre, Coppo, Emma, Johnson, Karpman, Landau, Langman, Lapeyraque, Licht, Nester, Pecoraro, Riedl, van, Van, Vivarelli and Frémeaux-Bacchi13]. The aetiology of aHUS is diverse and most often includes genetic mutations in the alternative complement pathway, increasingly a defining criterion for aHUS [Reference Loirat, Fakhouri, Ariceta, Besbas, Bitzan, Bjerre, Coppo, Emma, Johnson, Karpman, Landau, Langman, Lapeyraque, Licht, Nester, Pecoraro, Riedl, van, Van, Vivarelli and Frémeaux-Bacchi13, Reference Canpolat14]. In some instances, patients with aHUS report a diarrhoeal prodrome, which can obscure diagnosis. More recently, an aetiology-based classification has been adopted [Reference Michael, Bagga, Sartain and Smith15]. Infection-associated HUS largely equates to tHUS and includes eHUA, and pHUS. Atypical HUS (aHUS) is broadly used for all other types and can be classified as primary or secondary [Reference Michael, Bagga, Sartain and Smith15].
HUS is a rare development following STEC infection. In England, estimates from surveillance of STEC indicate that approximately 6.0% of STEC cases overall develop HUS [Reference Byrne, Jenkins, Launders, Elson and Adak16–Reference Adams, Byrne, Smith, Elson, Harris, Salmon, Smith, O’Brien, Adak and Jenkins18]. Children are most at risk of developing HUS following STEC infection, and it is the leading cause of intrinsic paediatric AKI in developed countries including the UK and the USA. Numerous studies indicate that children younger than 5 years most frequently develop HUS [Reference Dundas, Todd, Stewart, Murdoch, Chaudhuri and Hutchinson2, Reference Proulx and Sockett5, Reference Lynn, O’Brien, Taylor, Adak, Chart, Cheasty, Coia, Gillespie, Locking, Reilly, Smith, Waters and Willshaw6, Reference Schifferli, von, Fontana, Spartà, Schmid, Bianchetti and Rudin11, Reference Elliott, Robins-Browne, O’Loughlin, Bennett-Wood, Bourke, Henning, Hogg, Knight, Powell and Redmond12, Reference Byrne, Jenkins, Launders, Elson and Adak16, Reference Launders, Byrne, Jenkins, Harker, Charlett and Adak17, Reference Micheletti, Lavoratti, Materassi and Pela19–Reference Jenssen, Hovland, Bangstad, Nygård, Vold and Bjerre27].
The British Paediatric Surveillance Unit (BPSU) enables surveillance of rare childhood illnesses in the UK and Ireland. This methodology has been used to conduct two previous prospective surveillance studies on paediatric HUS in the UK and Ireland, one from 1985 to 1988 [Reference Milford, Taylor, Guttridge, Hall, Rowe and Kleanthous28], and the other from 1997 to 2001 [Reference Lynn, O’Brien, Taylor, Adak, Chart, Cheasty, Coia, Gillespie, Locking, Reilly, Smith, Waters and Willshaw6]. Here, we present findings from the third such study, undertaken for cases diagnosed in hospitals between 1 October 2011 and 31 October 2014. Building on the methodology of the previous BPSU-HUS studies, this study aimed to provide robust estimates of paediatric HUS incidence in England, Wales, Northern Ireland, and Ireland, by using data linkage and case reconciliation with existing laboratory and surveillance systems.
Our study also aimed to identify changes in incidence in the decade since the previous BPSU-HUS study and to establish characteristics of the condition, and current clinical management approaches used to treat HUS. Reconciliation of data captured through existing surveillance systems for STEC enabled surveillance to be evaluated for ascertainment of HUS and creation of a paediatric cohort to assess a range of predictors of HUS development already published elsewhere [Reference Adams, Byrne, Rose, Adak, Jenkins, Charlett, Violato, O’Brien, Whitehead, Barr, Taylor-Robinson and Hawker29].
Methods
Data collection
The British Paediatric Surveillance Unit research methodology was used to obtain HUS case notifications through its ‘orange card’ surveillance system, on a monthly basis between October 2011 and October 2014. Each month, all consultant paediatricians participating in the BPSU scheme were sent an electronic reporting card and requested to denote if they have seen any patient with any of the listed conditions in the preceding month and return it to the BPSU.
The BPSU forwarded on details of paediatricians notifying HUS cases to investigators within the UK Health Security Agency (UKHSA, formerly Public Health England). Paediatricians were then sent a standardised case questionnaire for each notification. The questionnaire collected patient-identifiable information, their height and weight, symptoms, microbiological findings, information on exposures, travel, contacts people experiencing diarrhoeal illness in the two weeks prior to hospital admission, and the case outcome at discharge.
The questionnaire also captured data on the hospital of admission, dates, and any referrals to or from other hospitals. Where questionnaires were received and denoted patients were referred to specialist centres, but no questionnaire had been received from that centre, a questionnaire with the pre-populated case details was forwarded to the relevant hospital for completion. Reminders were sent at fortnightly intervals, and annual audits were undertaken to optimize response from participants.
Data handling
De-duplication
BPSU notifications and case questionnaire data were entered and stored securely in a designated Microsoft Access database (Microsoft Corporation). Case data were reviewed for duplication, and where multiple questionnaires were received for the same case, the questionnaire from a specialist centre was retained over the non-specialist centre, but data were extracted from both questionnaires to ensure data were most complete and most recent.
Linkage with other surveillance systems
For English cases, National Health Service (NHS) number was used to link their data to records within the National Enhanced Surveillance System for STEC (NESSS) held at the UKHSA [Reference Michael, Bagga, Sartain and Smith15]. For cases in Wales and Northern Ireland, cases were linked to data in the UKHSA Gastro Data Warehouse (GDW), which contains reference microbiology results pertaining to STEC in England, Wales, and Northern Ireland. This linkage, via NHS number, allowed verification of any microbiological results reported on the questionnaires and to compare ascertainment of HUS against STEC laboratory reporting. For cases in Ireland, the Health and Safety Executive’s (HSE) Health Protection Surveillance Centre (HPSC) reconciled case data with that obtained through their national surveillance registry of HUS.
Case definitions
Based on questionnaire data, cases were coded as HUS or a non-case using the criteria set out in the clinical case definition, and then the HUS classification criteria were used to further categorise any HUS cases. For both criteria, where data were ambiguous or did not meet the case definitions, these cases questionnaires were reviewed and assigned to a group by a consultant paediatric nephrologist.
Clinical case definition for paediatric HUS:
A child (aged <16 years of age) who has:
-
• Acute kidney injury (AKI) defined by oligoanuria and/or elevated creatinine for age
AND EITHER
Microangiopathic haemolytic anaemia (MAHA)
AND/OR
-
• Thrombocytopenia
WITHOUT septicaemia at presentation*, malignant hypertension, chronic uraemia, or primary vascular disease
*Patients developing septicaemia after STEC was isolated from their faecal specimens were included.
HUS classification:
Typical HUS (tHUS): An HUS case with preceding diarrhoea and/or evidence of STEC infection
OR
An HUS case with or without diarrhoea and STEC isolated from the faecal specimen.
Pneumococcal HUS (pHUS): An HUS case with evidence of pneumococcal infection.
NB. Not all cases testing negative for eHUS were tested for pneumococcal infection.
Atypical HUS (aHUS): All other HUS cases.
Data analyses
Incidence rates for tHUS were calculated using the Office for National Statistics 2011 census population estimates as the denominator in England, Wales, and Northern Ireland and the Republic of Ireland Central Statistics Office, adjusted for the surveillance period. Continuous data that did not follow a normal distribution were described as medians with interquartile ranges (IQRs). Means and proportions were compared using Fishers’ exact test. Incidence rate ratios (IRRs) were calculated to compare rates. Incidence by ethnicity was calculated for cases in England and Wales only as no denominator was available for Northern Ireland and the Republic of Ireland.
The Lincoln–Peterson Index of population size was calculated using tHUS case numbers ascertained through the BPSU methodology and NESSS in England and the HPSC surveillance in Ireland to estimate the number of tHUS cases overall [Reference Balasubramani, Nowalk, Clarke, Dauer, Silveira, Middleton, Yassin and Zimmerman30]. The index was calculated as N= Cases captured by BPSU x Cases captured by NESSS (or HPSC surveillance in Ireland)/Cases captured by both BPSU and NESSS (or HPSC surveillance in Ireland).
Microbiological methods
In England, all faecal specimens from patients presenting to primary healthcare with suspected gastrointestinal infection are submitted to local hospital laboratories and tested for a range of gastrointestinal pathogens, including E. coli O157, following the UK Standards for Microbiology Investigations (https://www.gov.uk/government/collections/standards-for-microbiology-investigations-smi). Isolates identified as presumptive STEC O157 are then submitted to the PHE Gastrointestinal Bacteria Reference Unit (GBRU) for confirmation by PCR targeting stx1 and stx2 [Reference Byrne, Jenkins, Launders, Elson and Adak16, Reference Jenkins, Lawson, Cheasty and Willshaw31]. Diagnostic laboratories were also requested to refer stool specimens to GBRU, where no gastrointestinal pathogens including E. coli O157 were identified, for detection of non-O157 STEC, which cannot be cultured using standard protocols in the UK [Reference Jenkins, Lawson, Cheasty and Willshaw31, Reference Byrne, Vanstone, Perry, Launders, Adak, Godbole, Grant, Smith and Jenkins32]. Where it was not possible to obtain a faecal specimen, serum samples could be collected and were sent directly to the GBRU for testing for serum antibodies to lipopolysaccharide of E. coli O157 and a limited set of other E. coli serogroups (O26, O55, O103, O111, O145) [Reference Byrne, Chart and Cheasty33].
Results
Response rate
Between 1 October 2011 and 31 October 2014, 597 Orange Card notifications of HUS were made to the BPSU. After accounting for notification errors, duplication, and comparison to the clinical case definition, a total of 288 HUS cases were reported during the study.
Of the 597 Orange card referrals, notification errors were reported for 113 (18.9%) referrals. A questionnaire was returned from the reporting clinician for 394 of the remaining 484 valid notifications, giving an overall response rate of 81.4%. The response rate varied by country and was the highest from clinicians in Wales (93.3%) and the lowest from clinicians in Ireland (63.3%). After de-duplication, completed questionnaires were returned for 296 patients. For 15 cases (5.1%), the data collected were insufficient to categorise these cases, so were reviewed by a consultant paediatric nephrologist.
Overall, 288 of the 296 notified cases (96.6%) met the clinical case definition for HUS (breakdown by country in Table 3). Most (n = 256, 88.9%) HUS cases were typical, 22 (7.6%) atypical, and ten (3.5%) were pneumococcal HUS cases.
Where recorded, the specific laboratory investigations used to define HUS varied amongst cases (Table 1). All 288 HUS cases had evidence of AKI indicated by oligouria or anuria (n = 208) and/or elevated creatinine levels (n = 283), and 216 of 252 (85.7%) cases with complete data presented with the full triad of indicators for AKI, MAHA, and thrombocytopenia. Of the remainder (n = 30), 15 cases were not thrombocytopenic, and 22 lacked criteria for MAHA. Of the latter, 17 had low haemoglobin levels but absence of erythrocyte fragmentation, and five cases were classed as having normal haemoglobin, but all close to the cut-off of <10g/dL- between 10 and 11 g/dL.
Table 1. Presence and absence of diagnostic criteria for acute kidney injury (AKI), microangiopathic haemolytic anaemia (MAHA), and thrombocytopenia (T) amongst cases of haemolytic uraemic syndrome (HUS)a

a Excludes 34 cases with incomplete data recorded.
Case ascertainment
Comparison of the BPSU cases to existing NESSS data indicated an additional 166 HUS cases were captured through this study. A large proportion of those cases (148/166) were known to the NESSS as STEC cases but not confirmed as HUS cases (Figure 1). Reciprocally, in England, 25 paediatric HUS cases were reported in NESSS, which were not captured via this BPSU study, while HPSC reported 42 cases of HUS were not reported through the BPSU study.
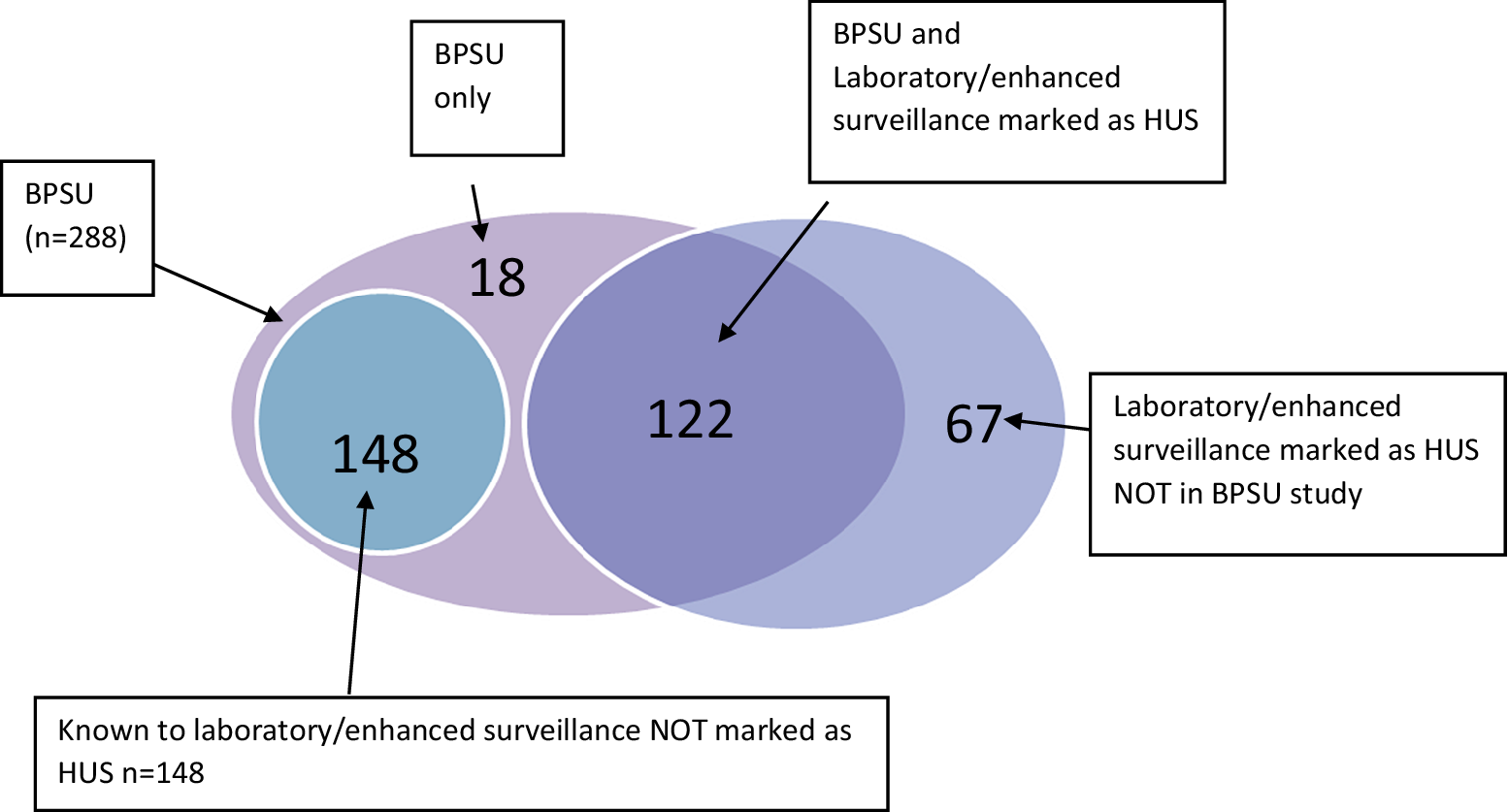
Figure 1. Venn diagram of HUS cases reported through the BPSU HUS study, the National Enhanced Surveillance System for STEC (NESSS), and the HPSC HUS surveillance register, 1 October 2011 to 31 October 2014 (n = 288).
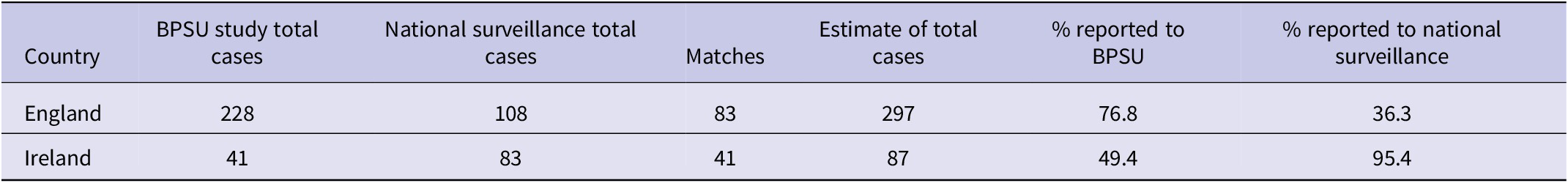
Lincoln–Petersen capture–recapture estimated an additional 44 HUS cases in England and two in Ireland, not captured either through this study or the existing surveillance systems. In England, the BPSU study was estimated to ascertain 76.8% cases but was lower in Ireland at 49.4% (Table 2).
Table 2. Completeness of reporting of HUS cases in England and Ireland reported through the BPSU study and through other surveillance systems (NESSS and HPSC)
Incidence and demography
The crude incidence of paediatric HUS was 0.78 per 100,000 person-years, although this varied by country, age, gender, and ethnicity.
Incidence was the highest in Ireland at 1.29 per 100, 000 person-years and was higher than that in the other countries (Table 3).
Table 3. Number of cases and incidence of HUS/100,000 person-years by country of residence reported through the BPSU HUS surveillance study, 1 October 2011 to 31 October 2014 compared to the 1997-2001 BPSU HUS surveillance study

Age was reported for 287/288 HUS cases, and the median age of cases was 3 (range 0-15. IQR 2.7) years. The majority (n=154, 53.7%) of cases were aged 1 to 4 years, and frequency decreased with subsequent increase in the age group (Figure 2). Incidence was higher amongst children aged 1–4 years than in all other age groups. Females were over-represented in the dataset with 157/288 cases (54.5%). However, proportionally, cases in the different age groups were similar for both sexes, except those aged 10-15 years, with a higher proportion of females than males (17.8% versus 6.9%), and a higher incidence rate in females than males in that age group (IRR:3.2,).

Figure 2. Number of haemolytic uraemic syndrome cases and incidence of HUS/100 000 person-years by age group and gender reported the BPSU HUS surveillance study, 1 October 2011 to 31 October 2014.
There were no notable differences in incidence by sex in the different countries, and incidence was the highest amongst 1- to 4-year-old children in all countries, except Ireland, where it was the highest in infants younger than 1 year.
Ethnicity of cases was reported for 277/288 cases. The majority (n = 253, 91.3%) were of any white background, and amongst other ethnic groups, Pakistani was the most frequently reported, with seven cases (2.5%). The incidence amongst white cases in England and Wales was 0.79 per 100,000 person-years.
Microbiological findings
Amongst the tHUS cases, microbiological evidence of STEC infection was documented for 221/256 (86.3%), STEC was isolated from 166 (64.8%) cases, and two cases tested positive for the stx gene by PCR in stools, but STEC was not isolated. A further 53 cases had antibodies to the somatic (O group) antigen of E. coli serogroups commonly associated with the STEC pathotype. Where STEC was isolated, 141 strains were STEC O157 and 11 were STEC O26. For STEC O157, 137 isolates were phage-typed, and the most frequent type (n=81) was PT21/28. Where toxin type was available (n=142), the most (n=134) strain was Stx2 only, with just eight strains that were Stx1+2.
The remaining 35 tHUS cases had a diarrhoeal prodrome, but a specimen was not submitted for testing (n=7), where serum samples only were provided and were negative for antibodies to E. coli O157 and the other five serotypes examined (n=17), or faecal specimens were provided but STEC was not detected (n=10). Lastly, for one case, E. coli O157 was detected but was stx negative.
Clinical features
Amongst 256 tHUS cases, 247 (96.5%) had a diarrhoeal prodrome, 184 (71.9%) of which had bloody diarrhoea, frequently accompanied by abdominal pain (n=175) and fever (n=106). The median time from onset of diarrhoeal symptoms to hospital admission or specific HUS diagnosis was 6 (IQR: 4-6) days. Three of the aHUS cases also reported a diarrhoeal prodrome.
Most (202/256, 78.9%) cases were oligouric or anuric. In total, 142 (55.5%) cases had extra-renal complications of tHUS, most frequently abnormal liver function tests (n = 105, 41.0% cases), followed by seizures (n = 39, 15.2% of cases) and hypertension (n = 31, 12.1% of cases). Eighteen cases had secondary septicaemia, after STEC had been isolated from their faecal specimens. Although patients with septicaemia at presentation were excluded from the study, those who developed septicaemia after tHUS had been confirmed were included. Other complications, and/or underlying conditions, were rarer; five cases had influenza-like illness, six cases had cardiomyopathy, seven had diabetes, and four cases suffered from a major haemorrhage. All four cases who had a major haemorrhage had had seizures, and two were also hypertensive with cardiomyopathy. In addition to these complications, one case had reported peritonitis and another peritoneal leak. Most cases with extra-renal complications had multiple manifestations, usually abnormal liver function accompanied by hypertension (n = 20) and/or seizures (n = 19). More females than males had extra-renal complications, 57.2% versus 45.9% of female and male tHUS cases, respectively, although this was not statistically significant (p = 0.0784).
Medications
Twenty-five (9.8%) of the tHUS cases had been prescribed non-steroidal anti-inflammatory drugs (NSAIDS), most cases (n = 22) prior to hospital admission and development of HUS, two cases after development and diagnosis of HUS, and it was unknown when for one case. Where dates were available (n = 15), the median number of days prior to hospital admission for prescription of NSAIDs was 3 (range 0 to 27) days. Five cases were prescribed corticosteroids, one case two days prior to admission and diagnosis of HUS, while the other four cases were treated with corticosteroids between 2 and 16 days post diagnosis of HUS. Twenty-two cases (8.6%) were prescribed antimotility medication prior to hospital admission. Eight cases were reported to have taken anti-diarrhoeal medications prior to admission. Forty-nine cases were treated with anti-hypertensives, 45 after admission as part of treatment following diagnosis of HUS, including 20 where the case was reported as having hypertension.
In total, antibiotic prescription was reported for 100 cases (39.1%), of which 85 had microbiological evidence of STEC infection. Where dates of prescription were available (88/100), 31 were treated with antibiotics prior to hospital admission, 21 on admission and 36 post admission.
Outcomes
Overall, a quarter of tHUS cases (n = 64) were admitted to the paediatric intensive care unit (PICU), and this did not vary by age or gender. Fifty-nine percent (151/256) of tHUS cases received one or more types of renal replacement therapy (RRT) including peritoneal dialysis, haemodialysis, and/or haemofiltration. Almost all cases requiring RRT were oligouric or anuric (144/151, 95.4%).
A greater proportion of female tHUS cases required RRT than males (64.1% versus 52.3%), although the difference was not statistically significant (p = 0.0724). A significantly greater proportion of tHUS cases with extra-renal complications required RRT (73.2%) than those with no extra-renal complications (45.0%, p = 0.0001), indicating more severe clinical manifestations in those cases. The mean creatinine level amongst cases requiring RRT was 546 mg/dL (95%CI: 505,589), substantially higher than the mean of 179 mg/dL (95%CI:150,208) for cases not requiring RRT.
Overall, cases prescribed antibiotics were more likely to require RRT than those who were not prescribed antibiotics (75.0% versus 48.7%, p = 0.0001) (Table 4), especially when cases prescribed antibiotics on or after admission (p = 0.0001).
Table 4. tHUS cases requiring renal replacement therapy (RRT) by history of antibiotic prescription
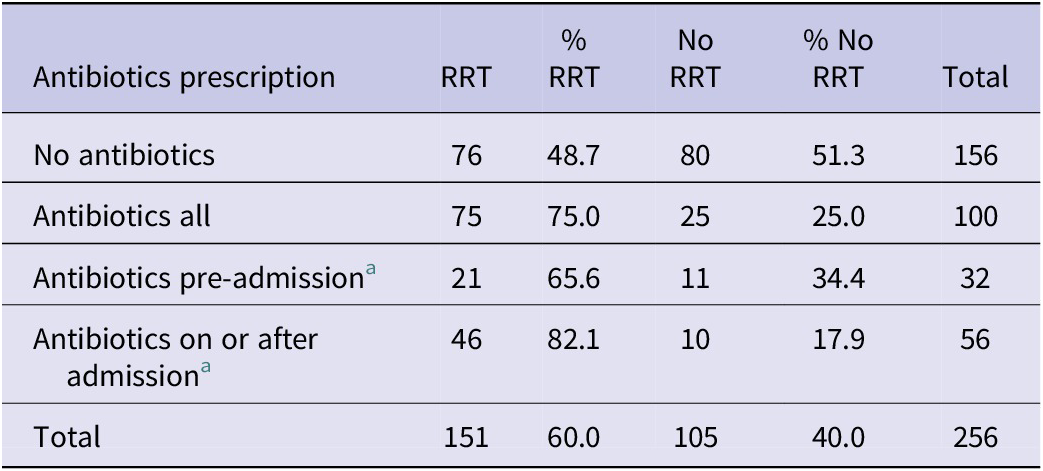
a Data on timing of antibiotic treatment were only available for 88 cases.
Data on outcome at discharge were completed for 239 (93.3%) tHUS cases. Clinicians reported 177 (74.1%) cases had made a seemingly full recovery when the questionnaire was completed. The most common complications at discharge were kidney damage (n = 48, four dialysis-dependent), hypertension (n = 15), and neurological impairment (n = 6). For 14 cases, hypertension alone was reported, and an additional two cases without renal damage were neurologically impaired. One patient died. While the recovery rate varied, more cases (79.5%) who did not require RRT fully recovered compared with cases who required RRT (60.8%). It also differed by oligouria or anuria, with only 64% oligouric or anuric patients making a full recovery compared with 84.4% with normal urine output.
Discussion
In our study, the response rate was high, and the BPSU methodology captured a substantial number of additional cases of HUS when compared to those captured through national STEC surveillance systems. Our study highlighted the limitations of the current surveillance system for STEC for monitoring the clinical burden of STEC and capturing HUS cases. Indeed, a cohort study using a linked dataset from the BPSU with data captured in NESSS indicated that compared to previous estimates of 6% STEC cases developing HUS overall and up to 15% in some paediatric groups [Reference Byrne, Jenkins, Launders, Elson and Adak16], the figure was as high as 19.5% in children [Reference Adams, Byrne, Rose, Adak, Jenkins, Charlett, Violato, O’Brien, Whitehead, Barr, Taylor-Robinson and Hawker29]. We concluded that the UKHSA NESSS was a sensitive system for monitoring cases of STEC in England but was less sensitive with respect to capturing clinical information and syndromic surveillance for cases of HUS. We speculate that this was due, for the most part, to the difficulties of linking laboratory and clinical information generated at different locations and times during the patient care pathway. The response rate from Irish paediatricians was considerably lower than that elsewhere, and capture–recapture estimated that only around a third of HUS cases in Ireland were reported, indicating that incidence was largely underestimated.
The annual incidence of 0.78/100,000 was comparable to reported rates elsewhere [Reference Elliott, Robins-Browne, O’Loughlin, Bennett-Wood, Bourke, Henning, Hogg, Knight, Powell and Redmond12, Reference Decludt, Bouvet, Mariani-Kurkdjian, Grimont, Grimont, Hubert and Loirat20, Reference Gerber, Karch, Allerberger, Verweyen and Zimmerhackl21], and there had been no notable changes in England, Wales, or Northern Ireland to previous estimates. In Ireland, however, incidence was estimated to have increased three-fold, and the exact reasons for this are unknown but coincide with an increase in reported STEC infections. Four decades since its emergence, STEC HUS has remained an important public health concern with no reduction in incidence observed over time. However, outcome data improved with one death (0.4%) reported here compared to fatalities of 5.6% and 1.8% in the 1980s and 1990s studies, respectively [Reference Lynn, O’Brien, Taylor, Adak, Chart, Cheasty, Coia, Gillespie, Locking, Reilly, Smith, Waters and Willshaw6, Reference Byrne, Jenkins, Launders, Elson and Adak16]. This figure is also lower than case fatality rates reported in comparable studies of between 2% and 4.6% [Reference Proulx and Sockett5, Reference Gerber, Karch, Allerberger, Verweyen and Zimmerhackl21, Reference Gould, Demma, Jones, Hurd, Vugia, Smith, Shiferaw, Segler, Palmer, Zansky and Griffin22, Reference Robitaille, Clermont, Mérouani, Phan and Lapeyraque34, Reference Mody, Gu, Griffin, Jones, Rounds, Shiferaw, Tobin-D’Angelo, Smith, Spina, Hurd, Lathrop, Palmer, Boothe, Luna-Gierke and Hoekstra35].
Concordant with other studies, incidence was the highest in those aged 1-4 years [Reference Lynn, O’Brien, Taylor, Adak, Chart, Cheasty, Coia, Gillespie, Locking, Reilly, Smith, Waters and Willshaw6, Reference Milford, Taylor, Guttridge, Hall, Rowe and Kleanthous28]. A number of studies reported an excess of female tHUS cases [Reference Ikeda, Ida, Kimoto, Takatorige, Nakanishi and Tatara3, Reference Proulx and Sockett5, Reference Launders, Byrne, Jenkins, Harker, Charlett and Adak17, Reference Micheletti, Lavoratti, Materassi and Pela19, Reference Gould, Demma, Jones, Hurd, Vugia, Smith, Shiferaw, Segler, Palmer, Zansky and Griffin22, Reference Cimolai, Carter, Morrison and Anderson25–Reference Milford, Taylor, Guttridge, Hall, Rowe and Kleanthous28, Reference Robitaille, Clermont, Mérouani, Phan and Lapeyraque34–Reference Rowe, Walop, Lior and Mackenzie37], and in some studies, a statistically significant association with female gender and developing tHUS was found [Reference Launders, Byrne, Jenkins, Harker, Charlett and Adak17, Reference Gould, Demma, Jones, Hurd, Vugia, Smith, Shiferaw, Segler, Palmer, Zansky and Griffin22, Reference Honda36, Reference Rowe, Walop, Lior and Mackenzie37]. A study in Japan demonstrated that in a post-mortem study, positive immunodetection of Gb3 was significantly more frequent in adult females than in males, and the study postulated that this could explain a gender disparity in risk [Reference Fujii, Mizoue, Kita, Kishimoto, Joh, Nakada, Ugajin, Naya, Nakamura, Tada, Okabe, Maruyama, Saitoh and Kurozawa38]. In our study, incidence was higher in females aged 10-15 than in males, although in a cohort study of STEC cases in England, after adjusting for other variables, females were not at increased risk of developing HUS amongst STEC cases [Reference Adams, Byrne, Rose, Adak, Jenkins, Charlett, Violato, O’Brien, Whitehead, Barr, Taylor-Robinson and Hawker29].
The gold standard for diagnosis of STEC-HUS is detection of the Shiga toxin gene by PCR, followed by isolation of STEC from a faecal specimen [Reference Jenkins, Byrne, Vishram, Sawyer, Balasegaram, Ahyow and Johnson39]. In our study, only 68% of tHUS cases had STEC isolated from a faecal specimen. Serology test results (specifically the detection of antibodies to the somatic (O group) antigen of E. coli serogroups commonly associated with the STEC pathotype) were available for a further subset of cases; however, this test does not detect antibodies to the Shiga toxin and therefore are not diagnostic for STEC-HUS. This gap in confirmation of culture-positive STEC via faecal samples restricts UKHSA’s ability to detect outbreaks, limits opportunities to implement health protection measures, and reduces the ability to determine the true burden of STEC and tHUS by different serogroups of STEC. The implementation of molecular assays in diagnostic laboratories starting in 2013 is improving timely diagnosis of STEC infection to inform clinical management of cases. In cases where a stool sample is not immediately available, a rectal swab taken prior to antibiotic administration and tested urgently for STEC by PCR and culture is recommended [Reference Jenkins, Byrne, Vishram, Sawyer, Balasegaram, Ahyow and Johnson39]. Early and accurate diagnosis facilitates prompt identification of patients who may benefit from treatment with eculizumab. Eculizumab is an expensive monoclonal antibody used long term to treat aHUS due to complement regulatory gene abnormalities. For tHUS, the treatment is best supportive care, focusing on renal and fluid replacement therapy. The treatment dichotomy for aHUS and tHUS therefore requires early diagnosis of, or ruling out of, STEC infection.
Where STEC was isolated, STEC serogroup O157 was most frequently reported (84.9%), and this is comparable to the 1997-2001 survey (83%). Furthermore, PT21/28 was the most common STEC O157 strain as per the 1997-2001 study. Most isolates were stx2 positive, consistent with the literature, which indicates that stx2 is most often associated with developing HUS [Reference Byrne, Adams and Jenkins40, Reference Koutsoumanis, Allende, Alvarez-Ordóñez, Bover-Cid, Chemaly, Davies, De, Herman, Hilbert, Lindqvist, Nauta, Peixe, Ru, Simmons, Skandamis, Suffredini, Jenkins, Monteiro, Morabito, Niskanen, Scheutz, da, Messens and Bolton41]. Most tHUS cases had a diarrhoeal prodrome, although only 71.9% had BD. While guidance exists for the management of acute bloody diarrhoea in children, HUS can develop in the absence of bloody diarrhoea, highlighting the importance of STEC testing, rather than reliance upon a diarrhoeal history for the differential diagnosis of aHUS and tHUS [Reference Jenkins, Byrne, Vishram, Sawyer, Balasegaram, Ahyow and Johnson39].
Antimicrobial therapy was reported for almost 40% cases in this study, and most often prescribed on or after admission to hospital. This is not surprising as sepsis is one of the most frequent differential diagnoses in cases presenting with symptoms of HUS, and the first dose of antibiotic is administered swiftly according to NICE sepsis guidelines (https://www.nice.org.uk/guidance/ng51/resources/sepsis-recognition-diagnosis-and-early-management-pdf-1837508256709). However, the use of antibiotics in STEC patients is controversial. A number of studies have suggested that antibiotic use amongst cases of STEC is a predictor for the development of tHUS [Reference Dundas, Todd, Stewart, Murdoch, Chaudhuri and Hutchinson2, Reference Launders, Byrne, Jenkins, Harker, Charlett and Adak17, Reference Pavia, Nichols, Green, Tauxe, Mottice, Greene, Wells, Siegler, Brewer and Hannon24, Reference Rowe, Walop, Lior and Mackenzie37, Reference Butler, Islam, Azad and Jones42–Reference Kawamura, Yamazaki and Tamai45]. Conversely, other studies have found no association with the development of tHUS and antibiotic usage [Reference Bell, Griffin, Lozano, Christie, Kobayashi and Tarr1, Reference Tserenpuntsag, Chang, Smith and Morse26, Reference Robitaille, Clermont, Mérouani, Phan and Lapeyraque34, Reference Jenkins, Byrne, Vishram, Sawyer, Balasegaram, Ahyow and Johnson39, Reference Butler, Islam, Azad and Jones42], and a minority report a protective effect of some antibiotic treatments of STEC cases. We were unable to examine further any effects of the use of antibiotics because indications and details on the timing were lacking. For example, it is unclear from the data if antibiotics were used initially and then ceased upon diagnosis of tHUS or if they were used as treatment for tHUS. It is clear that robust studies are needed to assess whether certain antibiotics may reduce severity and length of symptoms and whether antibiotics, if prescribed at certain points during illness, can be indicated.
Treatment with anti-diarrhoeal or antimotility drugs is also believed to increase the risk of developing tHUS because they slow motility through the gastrointestinal tract, leaving toxins in contact with the gastrointestinal mucosa for longer. A few studies identified the use of these drugs as predictors for developing tHUS [Reference Bell, Griffin, Lozano, Christie, Kobayashi and Tarr1, Reference Cimolai, Carter, Morrison and Anderson25, Reference Robitaille, Clermont, Mérouani, Phan and Lapeyraque34], while others have found no association [Reference Dundas, Todd, Stewart, Murdoch, Chaudhuri and Hutchinson2, Reference Launders, Byrne, Jenkins, Harker, Charlett and Adak17, Reference Butler, Islam, Azad and Jones42–Reference Kawamura, Yamazaki and Tamai45]. Non-steroidal anti-inflammatory drugs (NSAIDs) are nephrotoxic, and their use is an important cause of acute kidney injury in children [Reference Zoufaly, Cramer, Vettorazzi, Sayk, Bremer, Koop, de, Schmiedel, Jordan, Fraedrich, Asselborn, Nitschke, Neumann-Grutzeck, Magnus, Rüther, Fellermann, Stahl, Wegscheider and Lohse46]; however, only two studies have examined NSAID prescription and the development of tHUS and both found no association [Reference Misurac, Knoderer, Leiser, Nailescu, Wilson and Andreoli47, Reference Garg, Suri, Barrowman, Rehman, Matsell, Rosas-Arellano, Salvadori, Haynes and Clark48]. In our study, prescription of NSAIDs and antimotility drugs was reported relatively infrequently, and a cohort study would be more appropriate to assess any elevated risk associated with their administration.
The interpretation of studies on long-term outcomes of tHUS are problematic as historically they have included all classes of HUS and sometimes other microangiopathic anaemias. Many are small cohorts and had short follow-up periods, varied treatments, and different outcome measures. One systematic meta-analysis indicated that 25% of cases are left with chronic sequelae including a reduced glomerular filtration rate (GFR, hypertension or proteinuria, and a pooled incidence of 12% of cases with end-stage renal disease at follow-up [Reference Zoufaly, Cramer, Vettorazzi, Sayk, Bremer, Koop, de, Schmiedel, Jordan, Fraedrich, Asselborn, Nitschke, Neumann-Grutzeck, Magnus, Rüther, Fellermann, Stahl, Wegscheider and Lohse46]. Increased severity of kidney injury in acute illness, indicated by oligouria or anuria and requiring renal replacement therapy, and neurological complications, is strongly indicative of worse prognosis [Reference Schifferli, von, Fontana, Spartà, Schmid, Bianchetti and Rudin11, Reference Milford, Taylor, Guttridge, Hall, Rowe and Kleanthous28, Reference Byrne, Vanstone, Perry, Launders, Adak, Godbole, Grant, Smith and Jenkins32, Reference Misurac, Knoderer, Leiser, Nailescu, Wilson and Andreoli47–Reference Picard, Burtey, Bornet, Curti, Montana and Vanelle49]. Early fluid management and volume expansion in the period between diarrhoeal onset and development of HUS can prevent development of oligouria or anuria, more severe course of HUS [Reference Oakes, Kirkham, Nelson and Siegler50–Reference Balestracci, Martin, Toledo, Alvarado and Wainsztein52], and therefore long-term sequelae and death. In our study, consistent with the literature, cases who were oligouric or anuric less often made a full recovery and more often needed RRT [Reference Smith, Wilker, Reiter, Hedican, Bender and Hedberg53–Reference Hickey, Beattie, Cowieson, Miyashita, Strife, Frem, Peterson, Butani, Jones, Havens, Patel, Wong, Andreoli, Rothbaum, Beck and Tarr55].
In our study, tHUS was clinically significant and was frequently multisystemic, with over half cases experiencing extra-renal complications and 59.0% requiring dialysis. While only one death was reported, lower than both the previous paediatric studies in the UK, other sequelae were relatively frequent with only 69% cases making a full recovery.
Due to the high morbidity of HUS in children, the continued monitoring and control of STEC remains a high public health priority. Strengthening existing surveillance systems, including maximising diagnosis of STEC from stool specimens, will enable the relative contribution to severe disease by serogroup and Stx type to be better understood and improve outcomes.
Data availability statement
The data that support the findings of this study are provided in this article and are available from the UKHSA ([email protected]). If required, additional data may be available from the corresponding author with the permission of PHE. Restrictions apply to the availability of these data, which were used under licence for this study. Restrictions apply to the availability of data linked to patient information.
Acknowledgements
We would like to acknowledge the major contribution made to this study by Goutam Adak and Kirsten Glen.
Author contribution
Conceptualization: L.B., N.L., R.L., C.J.; Data curation: L.B.; Formal analysis: L.B., A.D., N.L.; Investigation: L.B., C.I., N.L.; Project administration: L.B., N.L., R.L.; Writing – original draft: L.B., C.J.; Supervision: G.G., N.L., R.L.; Writing – review & editing: G.G., A.D., C.I., N.L., C.J.; Validation: C.I.; Resources: R.L.
Financial support
This study was supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Gastrointestinal Infections, a partnership between the UK Health Security Agency, the University of Liverpool, and the University of Warwick. The views expressed are those of the author(s) and not necessarily those of the NIHR, the UK Health Security Agency, or the Department of Health and Social Care.
Competing interest
The authors declare none.
Ethical standard
This study was approved by the London–Camberwell St. Giles Research Ethics Committee (reference: 11/LO/1412).
Key findings
-
• This study captured a substantial number of additional cases of HUS when compared to those captured through national surveillance systems.
-
• We found 19.5% of children infected with STEC in England developed HUS compared to 6%, based on routine surveillance data.
-
• No notable changes to the annual incidence (0.78/100,000) in England, Wales, or Northern Ireland to previous estimates, although in Ireland, the incidence increased three-fold.
The fatality rate improved from 5.6% and 1.8% in the 1980s and 1990s to 0.4% in this study.









