Introduction
In recent years, there has been an increase in the incidence of waterborne infections internationally. This is mainly due to the ageing of water and sewage systems in some countries and possibly, also to improved laboratory methods used for the detection of waterborne pathogens [Reference Yang1–Reference Moreira and Bondelind3]. This increase has also been attributed to factors, such as overpopulation and urbanisation, mass migration and an increase of immunocompromised people in the population [Reference Eisenberg4, Reference Semenza and Menne5].
Waterborne infections are of great public health importance. They can appear as sporadic cases but can go on to affect many people in a short time [Reference Craun6–Reference Robertson8].
Most of the identified waterborne outbreaks (WBOs) in developed countries are caused by Campylobacter jejuni, norovirus, Cryptosporidium and Giardia lamblia [Reference Gallay7, Reference Jakopanec9–Reference Daly14].
Between 2005 and 2018 EU Member States and reporting EU non-Member States reported 286 WBOs affecting more than 54 609 people [15]. In Greece, 32 WBOs were recorded between 2004 and 2018. Seven of them had more than 200 recorded cases each. A large WBO challenges the health care system in different geographical regions of Greece every 2–3 years [Reference Karagiannis10, Reference Mellou16–Reference Vantarakis18].
On 26 January 2019, health care services in Northern Greece informed the National Public Health Organization (EODY) on the occurrence of a high number of gastroenteritis cases. The event attracted wide media and public attention.
This study aims to present the results of the outbreak investigation and summarise the particular characteristics and lessons learned from its management.
Methods
Setting
Town X has 8903 permanent residents (last census of 2011) and a dedicated water supply system that is distinct from those of neighbouring towns.
Epidemiological investigation
Descriptive epidemiology
We collected information from patients presenting at the local general hospital (GH) and the health care centre (HCC) with diarrhoea and/or vomiting with the onset of symptoms from 24 January 2019 to 04 February 2019. Demographic characteristics (age, sex), date of symptoms' onset, clinical manifestations (diarrhoea, vomiting, bloody diarrhoea and fever), hospitalisation and place of residence were collected using a structured form. Descriptive data were analysed to generate hypotheses on the possible source of the outbreak.
Case-control study
We performed an unmatched 1:1 case-control study (CCS) from the list of visitors at the HCC. As most recorded cases were residents of town Χ, the study population was restricted to the residents of the town. Moreover, the study population was restricted to the population aged over 16 years of age because of the lack of a paediatrician at the HCC, as minors aged between 16 and 18 years of age are treated by general practitioners like adults, in Greece.
We selected cases and controls randomly from the registry of the HCC since all visitors HCC – along with the reason for their visit – are recorded there.
We defined a case as any resident of the town, older than 16 years of age who visited the HCC between 25 and 28 January 2019 presenting diarrhoea and/or vomiting and control as any resident of the town, older than 16 years of age that visited the HCC between 25 and 28 January 2019 for reasons other than gastroenteritis symptoms, for example prescribing, scheduled appointments, diagnostic examinations.
A list of all cases with gastroenteritis symptoms and a list of controls were acquired, individuals were numbered and after creating random numbers with Microsoft EXCEL, cases and controls were randomly selected.
To develop the questionnaire, we conducted preliminary interviews with several cases on-site using generic questionnaires regarding their habits, recent activities, participation to events etc. Based on the collected information, we formed the hypothesis of the probable waterborne origin of the outbreak. Taking into consideration the information collected from these cases and local authorities and in accordance with the questionnaires used in the literature for the investigation of similar outbreaks, the questionnaire was finalised. Public health nurses of the HCC conducted face-to-face interviews with cases and controls after obtaining their verbal informed consent. Interviews took place from 31 January 2019 to 03 February 2019.
Information on demographic characteristics, date of symptoms' onset, duration of illness and absenteeism from work were included in the questionnaire. All participants were asked whether they had participated in a social event, whether they had consumed specific food items, tap or bottled water at home and at work, and the daily number of consumed water glasses. For each case and control, we recorded the number of household contacts and the number of contacts who developed gastroenteritis symptoms after the 24 January 2019.
Norovirus was detected in the first clinical specimens tested. As norovirus was suspected as the causative organism questions addressed to cases concerned a 2-day period prior to the onset of symptoms. Controls were questioned about possible exposures on 24–25 January 2019, as most cases had reported 26 January 2019 as the day of their symptoms' onset.
Cohort study
We conducted a retrospective cohort study (CS) among the students of the four elementary schools of the town.
We defined a case as any student aged 6–12 years who presented vomiting and/or diarrhoea from 25 to 28 January 2019.
A structured questionnaire, similar to the one used in the CCS, was distributed to the students on 30 January 2019, to be completed by their parents or guardians. We asked students to return questionnaires the following day. The questions concerned the 2-day period prior to the onset of the symptoms for cases and the period 24–25 January 2019 for non-cases.
Ethical issues
The study protocols for CCS and CS were reviewed and approved by the Scientific Board of EODY.
Interviews were conducted after informed consent was obtained.
The questionnaires used in both CCS and CS were developed by the investigation team consisting of epidemiologists from EODY that visited on-site and the local public health authorities and were approved by the Scientific Board of EODY.
All collected data were anonymised and entered in a database by the members of the investigation team at the EODY's premises. Data processing and analysis were managed in accordance with national and European Union laws.
Statistical analysis
We entered data in EpiData Manager statistical package and performed statistical analysis using Stata v12. We presented quantitative variables as means ± standard deviations or medians and interquartile ranges (IQR) and qualitative variables as absolute frequencies and percentages. Associations between the categorical variables and the occurrence of gastroenteritis symptoms were quantified using contingency tables with the calculation of chi-square test or Fisher's exact test. We calculated odds ratios (ORs) and 95% confidence intervals (CI) for the CCS and risk ratios (RR) for the CS. P-values <0.05 were considered as statistically significant. Stratification by consumption of tap water was conducted for variables statistically significant at univariate analysis.
Laboratory testing of clinical samples
We collected stool samples from patients with gastroenteritis symptoms that visited the health care services and sent to the Regional Public Health Laboratory (PEDY) of Thessaly. Samples were tested for bacteria (Salmonella spp., Shigella spp., Campylobacter spp., Yersinia enterocolitica, Escherichia coli O157) by standard culture methods.
All stool samples were also tested by polymerase chain reaction (PCR) for Campylobacter spp. Detection. Where non-fermenting sorbitol E. coli strains were isolated, H7:O157 serotype was confirmed by PCR, where fliCh7 and rfbO157 genes were targeted for the detection of O antigen and flagellar antigen of E. coli O157:H7. All non-fermenting sorbitol E. coli strains were tested for the presence of eae, stx1 and stx2 toxin-producing genes. For the nucleic acid extraction, the iPrepTM Invitrogen Purification Instrument (Thermo Fisher Scientific) with the iPrep PureLink Invitrogen Virus Kit (Thermo Fisher Scientific) was used and for the PCR assays the Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific).
Reverse transcriptase PCR (RT-PCR) for norovirus detection was performed, using the Super Script III One-Step qRT-PCR System with Platinum Taq Polymerase kit (Invitrogen). All PCR reactions were performed in a validated Applied Biosystems Verity 96 well Thermal Cycler. Norovirus positive samples were sequenced and sequence identity was determined using RIVM Noronet, Norovirus Genotyping Tool version 2.0 [19]. The primers and the PCR conditions used for the performed molecular analyses are provided in detail in Tables 1 and 2, respectively [Reference Sai20–Reference Harzandi23].
Table 1. Primers, their sequence and use for the laboratory investigation of the waterborne outbreak, town in Northern, Greece, January–February 2019
Table 2. PCR conditions for all molecular analyses performed during the investigation of the waterborne outbreak, town in Northern, Greece, January–February 2019
Stool samples were also tested using a multiplexed molecular assay, the BioFire FilmArray Gastrointestinal (GI) Panel, targeting 22 different pathogens in a single reaction; Enterotoxigenic E.coli (ETEC), Enteropathogenic E. coli (EPEC), Enteroaggregative E. coli (EAEC) and Shiga-like toxin-producing E. coli (STEC) with specific identification of E. coli O157, Enteroinvasive E.coli/Shigella (EIEC), Campylobacter (jejuni/coli/upsaliensis), Vibrio (parahaemolyticus/vulnificus/cholerae), Yersinia enterocolitica, Plesiomonas shigelloides, Clostridium difficile (Toxin A/B), Salmonella, Cryptosporidium, Cyclospora cayetanensis, Entamoeba histolytica, Giardia lamblia, Adenovirus F40/41, Astrovirus, Norovirus GI/GII, Rotavirus A and Sapovirus (genogroups I,II,IV and V) [24].
Environmental investigation and sampling
We collected information on the town's water supply, water chlorination and drainage system, as well as the proximity of livestock holdings to water abstraction points and storage and obtained the municipality chlorination records and records of technical works carried out at the water distribution system since the beginning of December 2018.
The local authorities performed water sampling from the water supply system on 25 and 26 January 2019. Local public health authorities also performed water sampling on 26 January 2019 from the system and one of the town's springs. All water samples were tested for microbiological indicators at PEDY; total aerobic count at 22 °C and 37 °C (ISO 6222:1999), number of total coliforms colonies per 100 ml and number of Ε. coli colonies per 100 ml (ISO 9308-01:2000), number of Enterococci spp. colonies per 100 ml (ISO 7899-02: 2000) and the number of Clostridium perfringens colonies per 100 ml (ISO 14189:2013).
In addition, 10 l of water sampled from the water supply system and the spring by the local public health authority on 26 January 2019 and were molecularly tested for norovirus at PEDY. The procedure for the concentration of the viruses was based on the elution of the viruses with glycine-alkaline buffer followed by organic flocculation with skimmed-milk, as described by Calgua et al. [Reference Calgua25]. After sample concentration, nucleic acid extraction followed using the iPrepTM Invitrogen Purification Instrument (Thermo Fisher Scientific) with the iPrep PureLink Invitrogen Virus Kit (Thermo Fisher Scientific) and subsequently, RT-PCR for norovirus detection was performed, using the Super Script III One-Step qRT-PCR System with Platinum Taq Polymerase kit (Invitrogen). Similar to the clinical samples, the PCR reactions were performed in a validated Applied Biosystems Verity 96 well Thermal Cycler. The primers used and the PCR conditions are included in Tables 1 and 2 [Reference Sai20–Reference Harzandi23].
On 5 February 2019, the local public health authority inspected the water supply system and measured the residual chlorine at the pumping station, the tanks and various points of the town's water distribution network. Local weather reports were reviewed for January 2019 [26].
Results
Descriptive analysis
In total, we recorded 638 gastroenteritis cases with symptoms onset from 25 January 2019 to 04 February 2019. Almost half of them (53%) were female and the median age was 44 years (range: 0–93). The majority (79%) were residents of town X. Τhe first gastroenteritis cases appeared early on Friday, 25 January 2019, with increasing numbers of cases recorded between 25 January 2019 and 29 January 2019. Most cases had an onset of symptoms on 26 January 2019. From 27 January 2019, numbers of cases decreased, with the last case occurring on 4 February 2019 (Fig. 1).
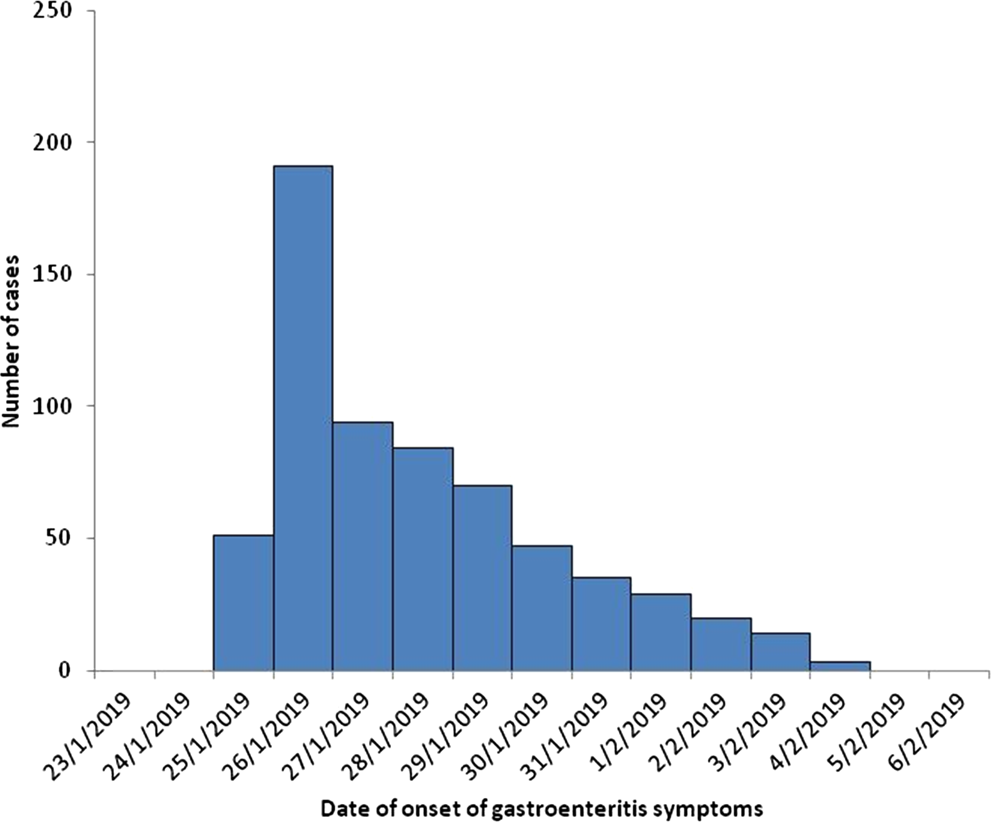
Fig. 1. Number of gastroenteritis cases by date of onset of symptoms (n = 638), health care centre and general hospital, January–February 2019.
The main reported symptoms were diarrhoea (89%) and vomiting (86%). Hospitalisation, mainly short stay, was required for 430 (80%) patients; 183 (43%) cases were hospitalised at the GH and 247 (57%) at the HCC to receive fluids intravenously usually for some hours. We did not record any death.
Case-control study
We selected 50 cases and 50 controls. We excluded two cases (4%) that did not meet the case definition. The median age of cases and controls was 51 (range: 23–78) and 56 (range:25–87) years old, respectively. Sex distribution among cases and controls was similar (45% and 55% of cases and controls, respectively, were females) (P-value = 0.3).
Cases reported diarrhoea (92%), fatigue (89%), vomiting (79%), arthralgia (77%), abdominal pain (68%), nausea (52%) and fever (>38 °C) (37%). The median duration of symptoms was 4 days (range: 3–5 days).
In total, 22 of the 31 (71%) cases, that the respective information was available, reported absence from work for at least 1 day (median: 2 days; range: 1–3 days).
According to the results of the univariate analysis, cases were more likely to consume tap water in the 2 days before symptoms onset (OR 10.6, 95% CI 2.20–99.0) and were more likely to use tap water to produce ice cubes (OR 3.7, 95% CI 1.24–12.0) (Table 3). A total of 45 of the 47 cases (96%) reported consuming tap water. Cases were less likely to consume bottled water (OR 0.16, 95% CI 0.02–0.80). No dose-response effect was observed and the likelihood of being a case did not increase with the number of glasses of tap water consumed. No food consumption or common activity had a statistically significant association with the occurrence of gastroenteritis symptoms.
Table 3. Results of the univariate analysis for the consumption of tap water, various foods and drinking water consumption estimators (proxies), case-control study, town in Northern, Greece, January–February 2019
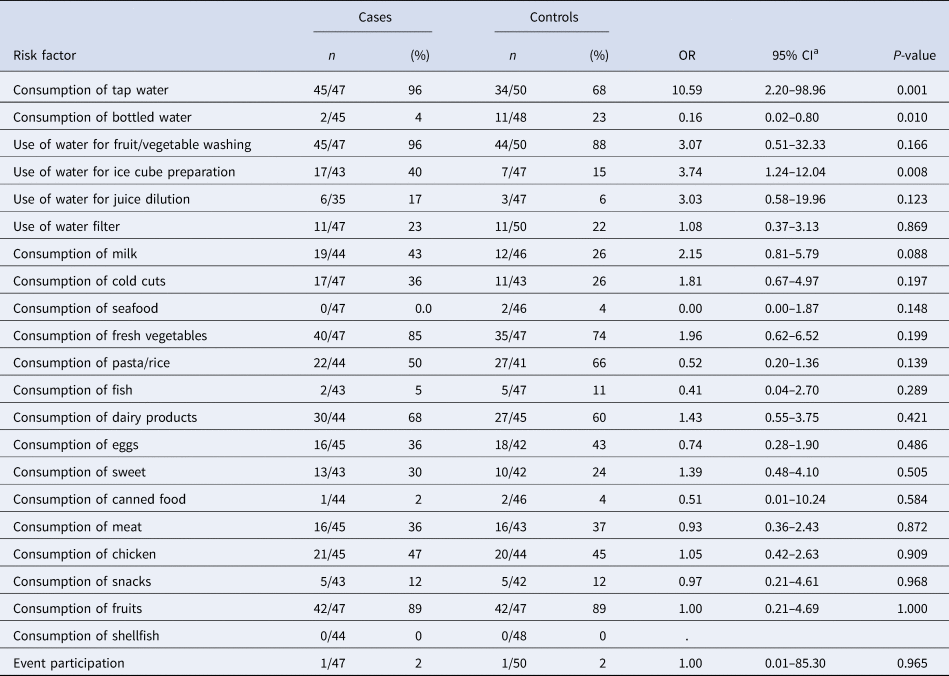
a OR, odds ratio; CI, confidence interval.
Stratification by consumption of tap water for bottled water and the use of tap water for making ice cubes did not show a confounding or effect modification.
Of the 137 persons who lived in the same house as a case, 79 (58%) also developed gastroenteritis symptoms at the time of the interview.
Cohort study
Of 452 students enrolled at the four schools studied, 236 participated in the study (response rate: 55%); 123 (53%) were girls and 123 met the case definition (attack rate: 53%). Age had a statistically significant association with being a case (P-value < 0.001).
Reported symptoms were vomiting (84%), abdominal pain (81%), fatigue (72%), diarrhoea (68%), nausea (54%), fever (>38 °C) (39%) and arthralgia (24%). The median duration of symptoms was 2 days (range: 2–3 days).
Of the total, 71% of the cases were absent from school for at least 1 day; the median duration of absence was 2 days (range: 1–3 days). The total number of lost school days among the 123 cases was 162.
In the univariate analysis, the tap water consumption within 2 days prior to the onset of the symptoms was associated with gastroenteritis (RR = 2.22, 95% CI 1.42–3.46) (Table 4). The consumption of bottled water had a negative effect (RR = 0.49, 95% CI 0.34–0.71). We were not able to detect an association between the daily number of consumed glasses of tap water and illness. No food consumption or activity was found to be associated with being a case.
Table 4. Results of the univariate analysis for the consumption of tap water, various foodstuffs and tap water estimators (proxies), cohort study, town in Northern Greece, January–February 2019
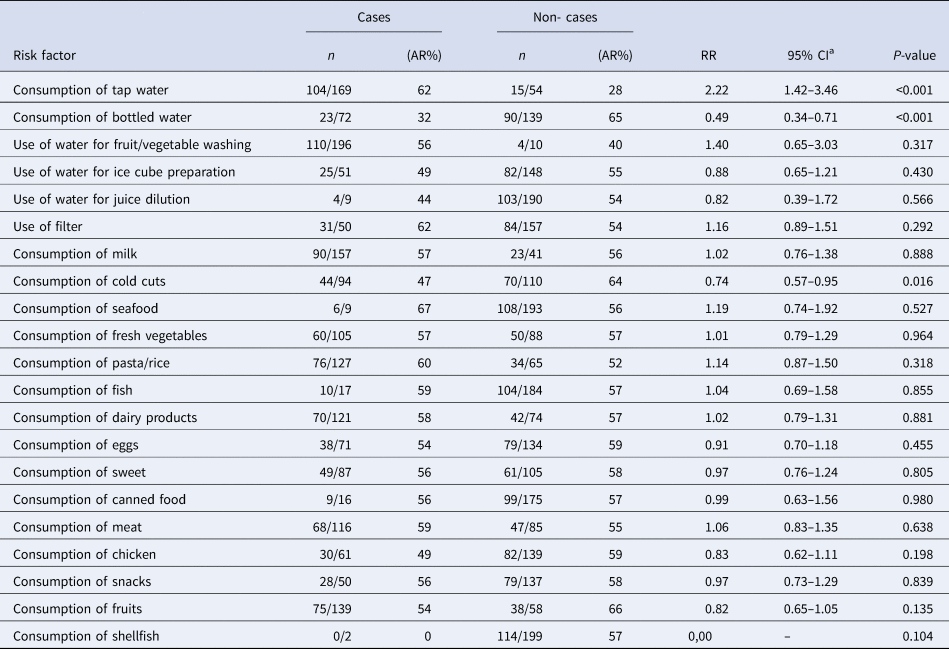
a RR, relative risk; CI: confidence interval.
After adjusting for age, the occurrence of gastroenteritis symptoms was statistically significantly associated with tap water consumption (OR 4.4, 95% CI 2.15–9.07).
Out of the 344 household contacts, 260 (76%) had also developed gastroenteritis symptoms by the time of responding to the questionnaire.
Laboratory results of clinical samples
More than one pathogen was detected in six of the 11 stool samples tested (Table 5); four were positive for two and two were positive for three pathogens each. Campylobacter spp. was the most commonly identified pathogen (found in eight clinical samples), followed by norovirus (found in five). Campylobacter spp. was detected together with various pathogens, mostly with diarrheagenic E.coli. Two norovirus strains were sequenced and belonged to the GII.Pe-GII.4_Sydney_2012 genotype (GenBank accession No MN923214-MN923215).
Table 5. Summary of laboratory results of stool samples collected from patients with gastroenteritis symptoms (n = 11), town in Northern Greece, January–February 2019
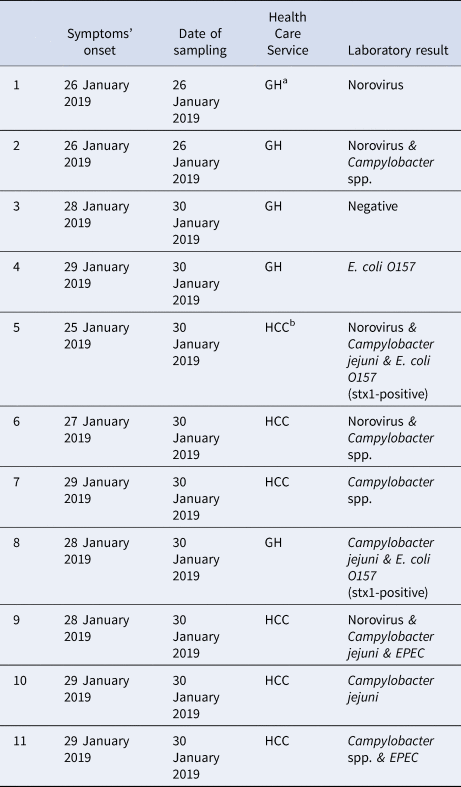
a GH, general hospital; bHCC, health care centre of the town.
Environmental investigation results
The springs supplying the town also provided water to other areas where gastroenteritis cases were not reported. From the springs, water is channelled to the autonomous water supply system of the town. This system consists of a pumping station where chlorination takes place. Four pumps fill two two-room tanks and a conduit, which is branched into two parallel conduits, spaced about 10 m apart.
Residual chlorine levels are monitored weekly by the municipality. From 01 December 2018 to 24 January 2019 residual chlorine ranged between 0.10 mg/l and 0.20 mg/l. On 25 January 2019, eight measurements of the residual chlorine were carried out, with values ranging from 0.10 mg/l to 0.50 mg/l. Four of these measurements were conducted before the ad-hoc chlorination that was ordered by the municipality authorities later on the same day. This response measure led to an increase of the residual chlorine content to 0.50 mg/l in the water reservoir for 24 h. However, when residual chlorine was measured at different points of the town's water supply system during the inspection by the public health inspectors, the values ranged from 0.07 mg/l (at a point of the water distribution network) to 0.31 mg/l (entrance of water in one of the tanks).
The low levels of residual chlorine at certain distribution points could not be supported by any record of missed chlorination events at the pumping station. Technical municipal records also did not report any malfunctions since early January 2019.
However, an inspection of the water supply system revealed technical failures; disintegration of the cement, especially on the tank roofs, a lack of a protective surrounding enclosure in one of the tanks and a lack of appropriate protective equipment on the windows of both the pumping station and the tanks.
Microbiological testing of water samples collected by the municipality were negative.
Six water samples were collected on 26 January 2019 by the public health inspectors after ad-hoc chlorination. The five samples collected from the water supply network were negative for all the indicators while the sample from the spring was positive for E. coli (<4 cfu/100 ml) and total coliforms (15 cfu/100 ml). Molecular testing of spring water for norovirus was negative.
Finally, there were no livestock holdings close to the springs or tanks' area and the wastewater pipeline was not in close proximity with the drinking-water pipeline. Almost all town residences were connected to the public sewage system. There was no recorded extreme weather event in the area in January 2019.
Discussion
Between 25 and 29 January 2019, we identified a sudden increase in the occurrence of gastroenteritis cases, with a peak on 26 January 2019 in a town of Northern Greece.
The results of the outbreak investigation point to the possible waterborne source. As well as epidemiological evidence, a waterborne source is also supported by the outbreak's size, the absence of a common activity or food item consumption among cases and the fact that there was no recorded increase of gastroenteritis cases in nearby areas/villages with a different water supply system.
The significance of the epidemiological investigation of this outbreak arises from the fact that laboratory confirmation was not possible.
In this outbreak, we recorded 638 cases. However, the actual burden of the disease was much higher given the estimated attack rate among students in the CS and the high proportion of symptomatic household contacts of cases in the CCS and the CS. High attack rates were recorded in previous WBOs, however, the total cost for the local community in such outbreaks cannot be easily estimated [Reference Karagiannis10, Reference Mellou16–Reference Vantarakis18, Reference Belliot27, Reference Widerström28]. In this outbreak, the hospitalisation rate and the number of consultations depicted the burden for the local HCSs, while absenteeism from work and school indicated a high indirect cost [Reference Baker29, Reference Chyzheuskaya30]. However, the overall duration of the outbreak was relatively short, possibly due to the implemented measures.
The identification of the mixed aetiology of the outbreak supported the hypothesis for possible faecal contamination of the water. Verification of the aetiological agent of WBOs is challenging as multi-pathogen outbreaks are common and adequate laboratory capacity for a variety of pathogens (bacteria, viruses, parasites) is required [Reference Craun6, Reference O'Reilly31, Reference Parasidis32]. In this respect, the added value of molecular methods for the detection of multiple pathogens in a single test, with the significantly reduced turnaround time for accurate results should be emphasised. Moreover, the high probability of mixed aetiology of WBOs and the different incubation period of the pathogens potentially involved may indicate the need to continue testing of samples even after the initial detection of a pathogen from the first clinical specimens.
Mixed origin is suggestive in that water may have become contaminated with faeces from sewage, rather than from a single person. Most large WBOs occur after environmental contamination of the water distribution system combined with the failure of chlorination systems and the inadequate maintenance of the system [Reference Moreira and Bondelind3, Reference Semenza and Nichols13]. The present outbreak investigation did not lead to a definitive conclusion on how water might have become contaminated and whether the faecal contamination was of human or animal origin. Technical failures related to the status of the water tanks and their enclosure allow us to assume that contamination of the water in the tanks due to the access of animals or unauthorised persons might be possible. Moreover, low levels of residual chlorine found during the inspection could possibly imply deficiencies in water sanitation practices.
The main limitation of the outbreak investigation was that laboratory investigation was not optimal overall. Water samples were collected after ad hoc chlorination and the waterborne origin of the outbreak was not confirmed. Furthermore, water samples were not tested for parasites due to the lack of a dedicated laboratory for testing of parasites in water samples. However, the patients' clinical manifestations and duration of symptoms were not compatible with parasitic infections [Reference Abeywardena, Jex and Gasser33].
In conclusion, this possible WBO demonstrates the impact of the contamination of the public water supply system on the local community and the health care services, the added value of analytical epidemiology in the investigation, the need for testing for a variety of pathogens when a waterborne source is suspected and the importance of having standard operational procedures for water sampling [34].
This outbreak led to an increased awareness in the local authorities on the measures needed to ensure water safety including upgrading the water supply system, continuous monitoring for early detection of faults, systematic water quality control appropriate treatment processes and protection during storage and distribution of water.
Acknowledgements
Dr Kassiani Mellou contributed equally to this article. The authors appreciate the contribution of all personnel who collected data used in this study. Special thanks to Mrs Eleni Lyllakou and Mrs Eirini Chinou, both working in the Department for Foodborne and Waterborne diseases of the National Public Health Organization, for their contribution in the recording of data in Epidata Manager statistical package. Additionally, many thanks to Professor Daniel Thomas, Consultant Epidemiologist at Public Health Wales for his valuable comments and contribution in the language editing. Finally, special thanks to the personnel of the health care centre of Argos Orestiko and the personnel of the public health authority of Kastoria for their support during the investigation.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interests
None.
Data availability statement
Data and other materials that would be necessary to replicate the findings of this article are available upon request at the corresponding author.









