INTRODUCTION
Hospitalizations for infectious diarrhoea comprise 2–4% of admissions in children aged <5 years in Australia [Reference D'Souza1]. Rotavirus is one of the most common causes of diarrhoea and one of the major causes of severe gastroenteritis in very young children [Reference Carlin2–Reference Konno7]. Many studies have shown that at least 30–60% of hospital admissions for acute gastroenteritis in young children are the result of rotavirus infection [Reference Carlin2–Reference Ryan11]. The rate of rotavirus diarrhoeal admission in children aged <5 years is in the range of 1·3/1000 in Canada [Reference Ford-Jones12] to 7·5/1000 children in Australia, and even higher in children aged <2 years (11·5/1000) [Reference Carlin2]. An estimated 10 000 hospital admissions occur annually in Australia for laboratory-confirmed rotavirus gastroenteritis [Reference Carlin2] and there are likely to be many more hospitalizations due to rotavirus that are coded as unidentified organisms. The direct cost of hospital care is estimated to be at least $Aus12 million and may be as high as $Aus15–18 million per annum [Reference Carlin2].
Rotavirus infection has a distinctive cyclical pattern with sharp peaks occurring in mid to late winter, which is unique to rotavirus among all know major pathogens associated with gastroenteritis [Reference Carlin2, Reference Donelli8, Reference Cook13, Reference Rytlewska14]. Rotavirus has been called the ‘democratic virus’ because a large proportion of children in the world are infected during the first few years of life, whether they are rich or poor or live in temperate or tropical areas [Reference Cook13].
Rotavirus can remain viable outside the human body from several hours to several months, depending on the environment. The ideal environment for its survival consists of low temperature (4–20°C); low pH (~3); and low humidity and protection from ultraviolet radiation. In faeces, rotavirus is found to be very stable at low and high relative humidity but not in the medium range of relative humidity. Rotavirus infectivity is lost more rapidly at 37°C than at 4°C or 20°C [Reference Moe and Shirley15]. Even at ambient temperatures above 30°C rotavirus particles stored in faeces are stable and may be infectious in vitro after 2·5 months of storage [Reference Fischer, Steinsland and Valentiner-Branth16].
There is evidence to suggest that the seasonal pattern of rotavirus infections may be related to climatic factors. Cold and dry weather have been associated with a higher number of rotavirus gastroenteritis hospital admissions, suggesting weather-related increases in exposure to rotavirus infection [Reference Brandt6, Reference Brandt17]. Indoor relative humidity has also been speculated to be an important factor for young infants [Reference Konno18, Reference Paul and Erinle19]. Other studies suggest that monthly rainfall might be one of the most important environmental determinants of rotavirus infectivity [Reference Cook13, Reference Konno18, Reference Doan20]. Rotavirus infection has been reported to occur more in the dry season compared to the wet season [Reference Doan20] in African countries like Morocco, Algeria, and Egypt [Reference Hieber21–Reference el-Mougi24], and in Bangladesh [Reference Black25] India [Reference Maiya26] and Costa Rica [Reference Hieber21].
By quantifying the relationship between climatic factors and rotavirus diarrhoeal admissions, data on season and recent climatic conditions can be used to make projections of the number of cases that are likely to occur and health services could be planned accordingly. The objectives of this paper are to investigate the seasonal variation of paediatric rotavirus diarrhoeal hospital admissions in three Australian cities and to quantify the relationship with temperature and humidity. These relationships are compared across cities.
METHODS
The study was conducted for three Australian cities – Brisbane (sub-tropical, coastal), Canberra (dry, inland and temperate) and Melbourne (temperate, coastal). For each of the three cities, data on daily admissions of rotavirus diarrhoea (International Classification of Diseases ICD-9: 008; ICD-10: A08) in children aged 0–5 years were obtained from the corresponding State Health Department, over the years 1993–2003. Daily temperature and relative humidity data from central monitoring stations in each city for the same years was obtained from the Bureau of Meteorology. Using data from the three cities provides the opportunity to pool data, thereby improving the precision of parameter estimates, and checking that effects found are consistent across cities having a wide range of climatic conditions.
For each city, daily admissions, daily average temperature and daily relative humidity for each week over the 11-year observation period were aggregated to weekly admission counts of rotavirus diarrhoea, average weekly temperature and humidity, for each city. The population aged <5 years for each city from the 1996 and 2001 census data was used to adjust for differences in population size of the three cities.
Seasonal patterns
To examine patterns over the observation period, the average weekly temperature, relative humidity and the weekly number of admissions of rotavirus diarrhoea were plotted over the 11-year period for each city. To display seasonal patterns, we aggregated the 11 years of data by week of the year (i.e. 1–52), against which we plotted average temperature and relative humidity, and the number of admissions, for each city.
Model for testing association between climatic factors (temperature and humidity) and rotavirus diarrhoea
The main objective of the analysis was to see whether temperature and humidity in the recent past are predictors of the number of admissions of rotavirus diarrhoea in children aged <5 years. The analysis we conducted is similar to that used for a study on the association of temperature and salmonella notifications [Reference D'Souza27]. We fitted a log-linear regression model with negative binomial errors, to allow for over-dispersion in the admission counts. Rotavirus diarrhoea is highly infectious among young children. Therefore, to account for the autocorrelation between the current week's admissions with admissions in previous weeks, admissions of the previous 3 weeks were included in the models as an autoregressive term.
Long-term trends over time
Plausible contribution to gradual changes in the number of admissions over time include non-temperature-related factors such as changes in the population size, changes in infectivity of rotavirus, changes in the coding of hospital admissions or changes to diarrhoeal management strategies between years, among others. To allow for such changes, we included a smooth long-term time trend in the log-linear regression model. We used a cubic polynomial to accommodate a nonlinear long-term trend. More specifically, the model containing only the long-term trend and the autoregressive term for admissions in the previous 3 weeks is

where time=week, which takes values 1, 2, …, 572. The ‘population size’ term, was accommodated by declaring the offset to be log (population size of children aged <5 years), which allowed comparison across cities of different size. As the reasons for the long-term trends are not identifiable, we have no basis for predicting the future direction of the trend. For this reason, we did not extrapolate beyond the boundary of the observation period. We did not explore the possibility that some of the long-term trend is due to longer-term change or variability in climatic factors, such as El Niño effects.
Seasonal effects and temperature effects
Log-linear models were fitted to all the data in each city separately to describe admissions. Explanatory variables included calendar time (1–572 weeks), previous 3 weeks' admissions, season, temperature and humidity. Calendar time accounted for long-term changes. The term ‘previous 3 weeks' admission’ reflects the serial interval of person-to-person transmission of the infection. Season had four categories of summer (December, January, February), autumn (March, April, May), winter (June, July, August) and spring (September, October, November). Four lags of temperature and humidity were tested and the previous week's temperature and humidity showed the strongest effect. The effect described by season may include temperature and humidity effects, but may also include effects due to seasonal changes in behaviour. Inclusion of climatic factors in the model are able to indicate an effect due to temperature and humidity that is over and above that accounted for by the season term. More specifically, our comprehensive model is
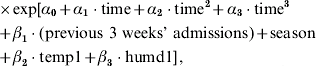
where temp1=mean temperature of previous week, humd1=mean humidity of previous week, and season=categorical variable indicating each of the four seasons. No coefficient is displayed for season because it is a categorical variable.
Effects of temperature and humidity on rotavirus diarrhoeal admissions in the three cities
The data for all cities were pooled together and interactions between temperature and city and between humidity and city were tested to investigate if there were significant differences in the effects of temperature and humidity on rotavirus admissions in the three cities.
Effect of temperature and humidity on rotavirus diarrhoeal hospital admissions within each season
To investigate whether the effect of temperature and humidity on rotavirus diarrhoeal admissions differed by season, we tested for interactions between season and temperature and between season and humidity. For each season separately, we also used the fitted Model 2 (without season) to predict admissions over a range of weekly mean temperatures and humidity in the three cities. To prevent different long-term trends from interfering with a comparison across cities, we fixed the ‘time’ variable at a value that reflects the recent past (we used December 2002 rather that than the most recent time because the fitted trend curves at the latter may be influenced by edge effects). Thus, predicted values can be thought of as predictions of current admissions. The estimated total number of admissions was calculated for each degree rise in temperature holding humidity constant. Similarly the number of rotavirus diarrhoeal admissions for a percent increase in humidity was calculated holding temperature constant.
RESULTS
Seasonal variation of hospital admissions of rotavirus diarrhoea
The population, latitude, admissions and rate of rotavirus admissions for the 11-year period for the three cities are shown in Table 1. Canberra is the smallest city, with just over 300 000 people, while Melbourne is the largest city, with over 3 million people. Rotavirus diarrhoeal admissions form 63% of all infectious diarrhoeal admissions in Canberra and only 38% in Melbourne. Similarly the rate of rotavirus diarrhoea admission rate among children aged <5 years was also significantly higher in Canberra (8·5/1000) compared to Melbourne (2·7/1000).
Table 1. Population, latitude, temperature and humidity range and rotavirus admissions in children aged <5 years for three cities (1993–2003)
Figure 1 shows the cyclical pattern of rotavirus diarrhoeal admissions over the 11-year period along with average weekly temperature and relative humidity. Seasonal patterns are shown in Figure 2 where the 11 years of data were aggregated by week and the average temperature, average humidity and the numbers of rotavirus admissions were plotted against week of the year, for each city (Fig. 2). The rise in admissions started around week 22 in Brisbane and Melbourne and a little later in Canberra (week 25). Admissions peaked in week 31 in Brisbane, week 34 in Melbourne and week 36 in Canberra. The increase in the number of rotavirus admissions between the seasonal low to high ranged from 157% to 310% in the three cities and was most marked in Canberra.
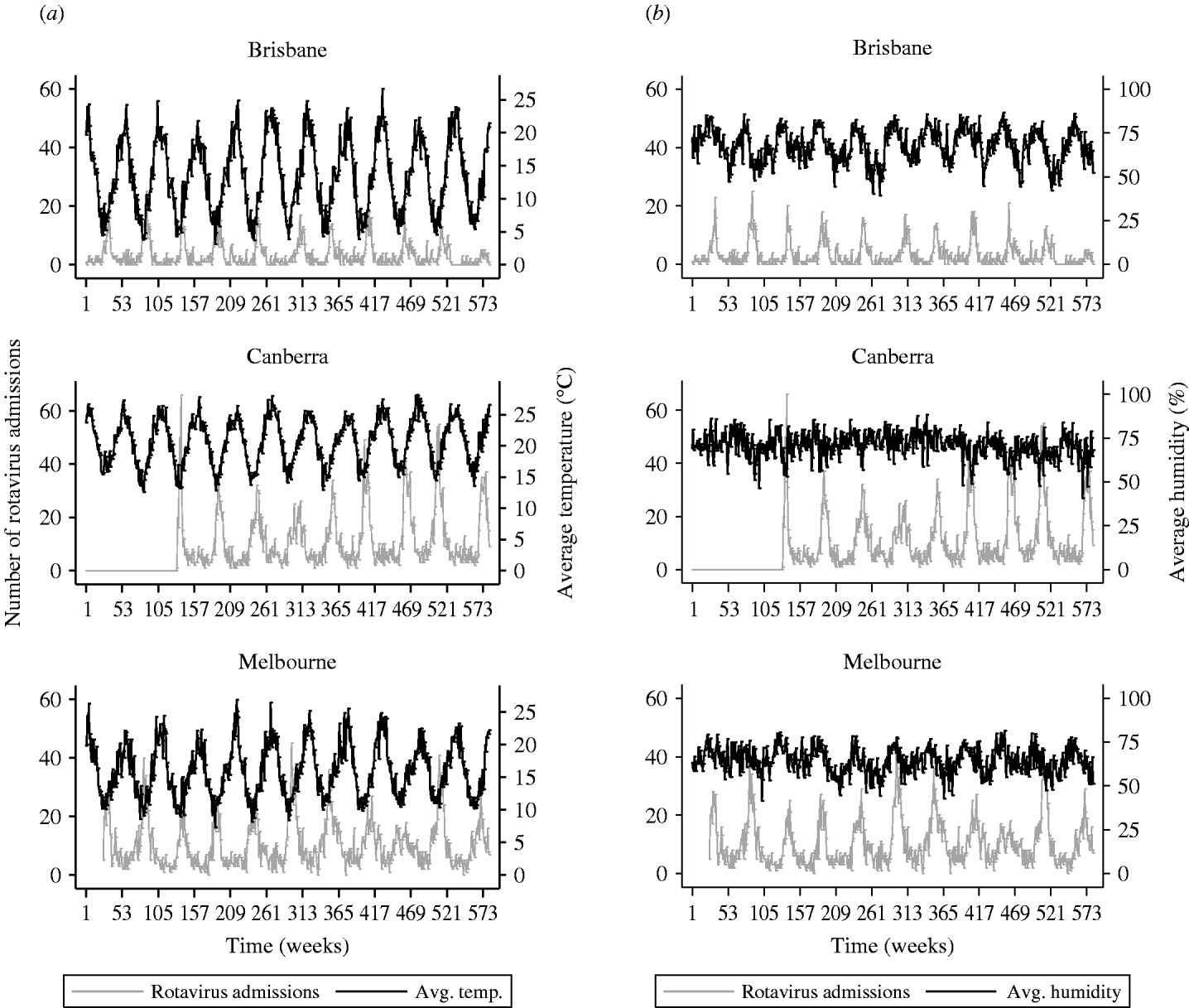
Fig. 1. Weekly number of rotavirus diarrhoeal admissions in children aged <5 years. (a) Mean weekly temperature (°C) and (b) mean weekly humidity (%) for three Australian cities, 1993–2003.
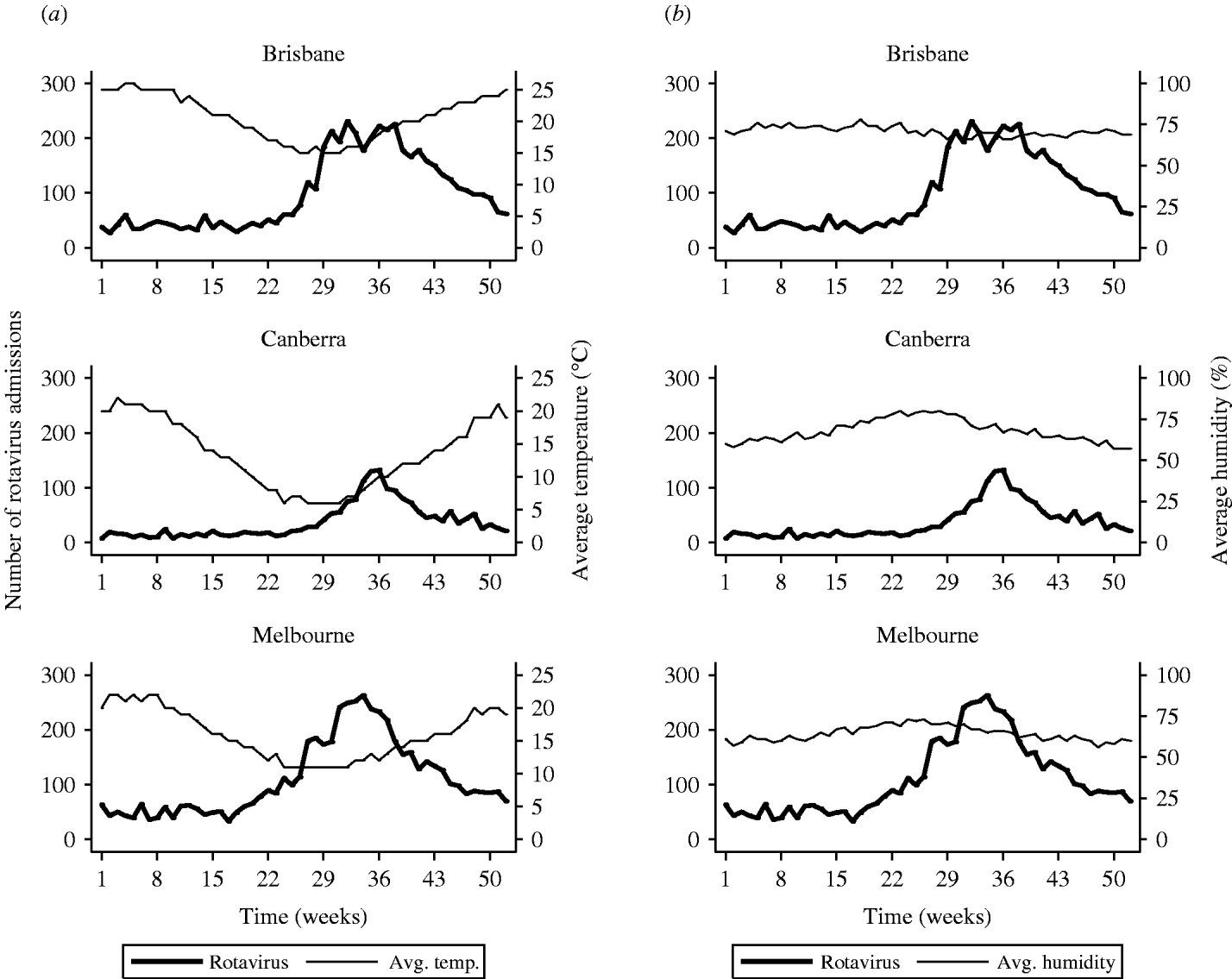
Fig. 2. Weekly number of rotavirus diarrhoeal admissions in children aged <5 years. (a) Mean weekly temperature (°C) and (b) mean weekly humidity (%) for three Australian cities, averaged for each week over 1993–2003.
In each city, as the temperature approaches the winter low point, the number of rotavirus admissions start to rise. The increase in admissions continues through the coldest months and into spring when temperatures are rising. In mid-spring the number of admissions suddenly starts to decline while temperatures continue to rise. Admissions reach a very low level in summer and throughout autumn. Humidity climbs slowly from summer into autumn. At peak humidity, just before winter, the admissions start to rise and continue to rise throughout winter while humidity continues to fall throughout winter and spring. The peak in rotavirus admissions occurs a number of weeks after humidity starts to decrease in all three cities.
Association between temperature and humidity with rotavirus diarrhoeal admissions
When Model 2 was fitted, an increase in the mean temperature of the previous week and an increase in the mean relative humidity were both statistically significantly associated with a decrease in the number of admissions of rotavirus diarrhoea (Table 2). The negative coefficient of temperature for rotavirus diarrhoea was largest in Canberra (≈−0·05, P<0·001) compared to Brisbane (≈−0·03, P<0·05) and Melbourne (−0·02, P<0·05). Relative humidity had the same effect in Canberra and Brisbane (−0·02, P<0·001) and a very small effect in Melbourne (−0·008, P<0·05). For a unit rise in the temperature the week before, there was a 5% decline in rotavirus diarrhoeal admissions in Canberra, 3% in Brisbane and 2% in Melbourne in the current week. An increase of 1% in relative humidity of the previous week was associated with a 2% decline in rotavirus diarrhoeal admissions in Canberra and Brisbane and a 1% decline in Melbourne in the current week. Although the coefficient looks small for humidity, it must be remembered that this is for a percent increase; and the range of humidity is quite wide and so the absolute effect is considerable. There was also a statistically significant positive association of the previous 3 weeks’ rotavirus diarrhoeal admissions with rotavirus diarrhoeal admissions in the current week.
Table 2. Regression coefficient, IRR and 95% CI for a 1 °C increase in temperature and 1% increase in humidity on admissions of rotavirus in children aged <5 years
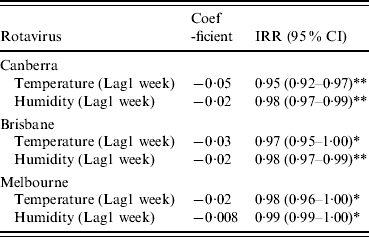
Adjusted for trend, season and previous 2 weeks' admission.
IRR, Incident rate ratio; CI, confidence interval.
* Significant at P<0·05, ** significant at P<0·001.
To explore the relationship further we added interaction effects to Model 2.
Effects of temperature and humidity on rotavirus diarrhoeal admissions in the three cities
There was a statistically significant interaction between temperature and city; indicating that the effect of temperature on rotavirus admissions was not the same across the three cities. In Canberra, a unit rise in temperature was associated with the greatest decrease in rotavirus admissions compared to Brisbane and Melbourne. There was no difference in the effect of humidity on rotavirus diarrhoeal admission in the three cities.
Effect of temperature and humidity within each season on rotavirus diarrhoeal admissions
The interaction between humidity and season was statistically significant in Brisbane (sharper decline in winter) and was of borderline significance in Canberra on rotavirus diarrhoeal admissions. In Brisbane, temperature showed statistically significantly different effects across seasons (an increase in autumn and no effect in the other seasons) but in Melbourne and Canberra there was no statistically significant different effect of temperature across the seasons.
Figures 3 and 4 show the number of rotavirus diarrhoeal admissions as temperature and humidity increased when separate models were fitted for each season. All other variables in the model were held constant for these plots. As temperature and humidity increased there was a reduction in rotavirus admissions in spring and winter in all three cities. In Melbourne, the effect of humidity on rotavirus diarrhoeal admissions was the same in all seasons while there was a larger negative effect in winter in Brisbane and in spring in Canberra (Fig. 4).

Fig. 3. Predicted values of rotavirus diarrhoeal admissions in children aged <5 years for an increase in mean of previous week's temperature for three Australian cities, 1993–2003, with time set at December 2002.

Fig. 4. Predicted values of rotavirus diarrhoeal admissions in children aged <5 years for an increase in mean of previous week's humidity for three Australian cities, 1993–2003, with time set at December 2002.
DISCUSSION
The overall seasonal pattern of rotavirus admissions in children aged <5 years observed in this study is consistent with seasonal patterns found elsewhere [Reference Carlin2, Reference Donelli8, Reference Cook13, Reference Rytlewska14]. Rotavirus admissions peaked in winter in Brisbane and Melbourne and in early spring in Canberra. The onset of cool and dry weather is associated with a sharp increase in rotavirus diarrhoeal admissions as temperature and humidity fall but then suddenly decline in mid spring. The decline in admissions may be due to a decline in the number of susceptible children in all three cities. A similar finding has also been seen among infants of Apache Indians living on desert reservations who experienced brief sharp outbreaks of rotavirus gastroenteritis soon after the onset of cool weather [Reference Engleberg28]. In northern Japan, rotavirus infection was found to appear abruptly when the mean temperature of any 10-day period became <5°C, reached a peak when it was <0°C and waned when it became >20°C [Reference Konno18].
The regularity of onset of increased rotavirus illness at about week 22, and the rise up to week 36 followed by a rapid decline, suggests ‘triggers’ that start the epidemic and end it. The timing is similar in two cities that have very different baseline climates and the absolute temperatures at the start of the rise and fall vary. Superimposed on this, temperature and humidity have an effect.
This is the first study to quantify an association between rotavirus hospital admissions in children aged <5 years and climatic factors. The time-series analysis showed that the risk of rotavirus diarrhoeal admissions had statistically significant negative relationships with average temperature and relative humidity of the previous week in all three cities when controlling for season. The effect of temperature and humidity on rotavirus diarrhoeal admissions varied significantly in different seasons. Temperature and humidity were important in winter and spring; in these seasons, colder temperature and lower humidity were associated with increased admissions for rotavirus diarrhoea. The period of autocorrelation was significant for rotavirus diarrhoeal admissions in the current week possibly reflecting the infectious nature of rotavirus and being spread from person to person.
Plausible reasons for association with climatic factors
The transmission of any infectious disease is necessarily complex, involving agent, host and environmental factors. Rotavirus cannot replicate outside the human body and animals are not normally involved in the transmission cycle. Rotavirus is transmitted from person to person via the faecal–oral route [Reference Leung, Kellner and Davies29] but more recently it has been recognized that it can be aerosolized [Reference Ijaz30] and the respiratory route has been implicated as a possible mode of transmission in humans [Reference Cook13]. The epidemiology of rotavirus with its increased incidence in the cooler months in both temperate and tropical climates in developed and developing countries resembles childhood viruses like measles that are spread by the respiratory route [Reference Cook13]. This might explain the winter seasonal peaks when respiratory infections also peak [Reference Brandt31]. In Washington, DC, the isolation of rotavirus in laboratories occurred with clockwork-like precision of the annual cycle, more so than other paediatric viruses, respiratory syncitial virus [Reference Kim32, Reference Parrott33] influenza A and B viruses [Reference Kim34] and the respiratory adenoviruses [Reference Brandt31].
Behaviour changes with season and people might spend more time indoors in cooler weather. Climatic factors may help in the survival and transmission of the rotavirus between human hosts. Along with this we might have a change in behaviour, such as people coming into closer contact with each other and thereby increasing the chance of person-to-person transmission. This can only be resolved in studies of households. The start of the rise in hospital admissions may also be due to host factors like lowered immunity or increased susceptibility in the colder months compared to warmer months.
It is possible that differences in rates of hospital admission for rotavirus diarrhoeal admissions reflect variations in patterns of primary care influencing the number of children who present to hospital emergency departments and/or differences in hospital admission policies, or might be due to variations in classification of discharge diagnosis of children admitted with acute gastroenteritis [Reference Ferson3].
This study has some limitations, as there may be some selection bias in using hospital admissions as people might differ significantly from those cases in the community. There may be a link with severity of disease and factors associated with weather. We were not able to control for housing, dietary habits, socioeconomic status, recent contact with other cases, indoor temperature and indoor relative humidity.
The predictable nature of excess rotavirus diarrhoeal admissions in winter and spring, together with an understanding of the links between climatic factors and rotavirus diarrhoeal disease, have the potential to predict demands on health service. If we know that there is a lag of at least 1 week between low temperatures and humidity and an increase in admissions, it could facilitate the appropriate reallocation of hospital staff and beds to prepare for seasonal fluctuations in cases, particularly as most or all of these admissions are emergency admissions and therefore unplanned.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
We thank the Information Management Unit of ACT Health, Victorian Health Department and Health Information Branch, Queensland Health for providing the hospitalization data. We also thank the Bureau of Meteorology for providing the temperature and humidity data for the same years. Financial support for this project was provided by the National Centre for Epidemiology and Population Health (NCEPH), The Australian National University. Rennie M. D'Souza was funded by NCEPH through a NHMRC Capacity building grant, Environment and Population Health: Research Development from Local to Global, 2003–2007 (No. 224215).








