INTRODUCTION
Guillain-Barré syndrome (GBS) is the most common form of acute flaccid paralysis (AFP) worldwide, with an estimated annual incidence of 1–2 cases/100 000 people in Western countries [Reference Sejvar1]. In the USA, GBS costs an estimated $1.7 billion annually, over US$300 000/patient [Reference Frenzen2]. Campylobacter jejuni infection, the most common cause of bacterial gastroenteritis in the USA [Reference Scallan3], is also the most commonly identified precipitant of GBS, preceding paralysis in about 30% of GBS patients [Reference Yuki and Hartung4]. GBS is estimated to follow 0·25–0·65/1000 cases of C. jejuni infection, with paralysis typically beginning 1–3 weeks after diarrhoea [Reference Yuki and Hartung4]. Several GBS subtypes are recognized, including acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN), and Fisher Syndrome (FS). The most common subtype in the USA and Europe is AIDP, accounting for about 90% of cases. However, AMAN predominates in other countries, including Mexico [Reference Nachamkin5]. C. jejuni infection is frequently associated with AMAN and FS [Reference Drenthen6, Reference Jacobs7].
Outbreaks of C. jejuni infection are uncommon and outbreaks of GBS rare. From 2003 to 2008, an average of 28 Campylobacter sp. outbreaks, mostly C. jejuni, per year were reported in the USA [Reference Taylor8]. A high incidence of Campylobacter sp. infection has been described in Mexican populations [Reference Calva9], but to our knowledge, no outbreaks have been reported, probably because of limited surveillance. C. jejuni outbreaks are commonly linked to poultry, unpasteurized dairy products, and drinking water [Reference Taylor8, Reference Engberg10–13]. No GBS outbreaks have been reported in Mexico, and only two have been reported in the USA: one associated with the national 1976 ‘swine’ influenza vaccine and a small cluster associated with evidence of recent cytomegalovirus infection [Reference Kaplan14–Reference Schonberger16].
On 16 June 2011, epidemiologists from the Mexican state of Sonora noted 15 cases of AFP reported in the previous 6 weeks in the border city of San Luis Río Colorado (SLRC). Public health officials in the USA also learned of four patients with GBS hospitalized in the US state of Arizona; two resided in SLRC and two in neighbouring Yuma County (YC), Arizona. A stool enzyme immunoassay (EIA) for Campylobacter sp. was positive in the one Arizona patient tested. On 28 June 2011, local, state, and federal health officials from the USA and Mexico launched a binational investigation, the first under a bilateral agreement complementing the new International Health Regulations (IHR) [Reference Kohl17]. The aims were to confirm and characterize the outbreak, evaluate possible precipitants of GBS, and determine the source in order to limit further illnesses.
METHODS
AFP surveillance and GBS cases
AFP is notifiable in Sonora; we reviewed the surveillance data. Neither AFP nor GBS is routinely notifiable in the USA; the Arizona Department of Health Services issued a health alert to clinicians on 25 June 2011, requesting notification of GBS cases. Based on Brighton Collaboration GBS case definitions [Reference Sejvar18], we classified AFP and GBS cases using information from medical records, Sonora AFP case report forms, clinician interviews, patient examinations, and electrodiagnostic studies. To investigate possible precipitants of GBS, including viral or bacterial infections or recent immunizations, we reviewed results of clinical tests, including those from commercial assays for IgG antibodies to C. jejuni, and interviewed patients regarding antecedent symptoms and exposures. We conducted additional case-finding through enhanced AFP surveillance in Sonora and outreach to Arizona clinicians.
Surveillance for Campylobacter sp. infection and diarrhoea
We reviewed culture-confirmed cases of campylobacteriosis in YC and other Arizona counties from January–June 2011. We reviewed surveillance data from Sonora, where syndromic surveillance for acute diarrhoeal illness is enhanced by stool culture for Salmonella enterica, Shigella sp., Escherichia coli, and Vibrio cholerae on a subset of cases. Campylobacter sp. diagnostics were not available in Sonora during the investigation.
Case-control investigation
After establishing that C. jejuni infection was associated with the outbreak, we conducted a case-control investigation from 8 to 26 July 2011, to identify sources. Eligible cases were GBS with Brighton level ⩾3 [Reference Sejvar18] or infection with the C. jejuni outbreak strain, with illness onset (paralysis or diarrhoea) from 15 April to 26 July 2011 in a person present at any time in YC or SLRC before illness onset during this period. Three controls per case were identified by systematic house-to-house recruitment, individually matched by age group (2–10 years, 11–25 years, >25 years), sex, ethnicity (Mexican descent or not), and neighbourhood of residence. The exposure period was 7–14 days before onset of paralysis for GBS case-patients without diarrhoea and 1 week before onset of diarrhoea for case-patients with diarrhoea. Controls were interviewed about the same week as their matched case regarding specific locations visited within YC and SLRC and, to decrease recall bias, about the period 1 week before interview regarding foods. Regarding water, controls were asked about both exposure windows.
Participants were asked whether each food item was eaten raw or cooked. For fruits and vegetables commonly washed with tap water and consumed raw (apples, cabbage, carrots, celery, cilantro, cucumbers, green onion, lettuce, onion, pears, spinach, strawberries, tomatoes), we compared the number reported by case-patients and controls. Items that respondents reported cooking were excluded.
Measures of association between exposures and illness were calculated by conditional logistic regression as matched odds ratios (mORs) with 95% confidence intervals (CIs) using maximum-likelihood estimates (MLEs), when available, and median unbiased estimates when MLEs could not be calculated. Medians were compared using the Kruskal–Wallis test. SAS version 9.3 (SAS Institute, USA) was used for analysis.
Environmental investigation
Between 1 July and 11 August 2011, we investigated drinking and wastewater systems, hydrology, and land-use practices. We plotted households with GBS patients on maps of drinking-water systems and overlaid water sectors categorized by the lowest residual chlorine level from routine household sampling within each sector from January to June 2011. We collected large-volume drinking-water samples (∼100 litres) using ultrafiltration (UF; without the use of sodium polyphosphate) [Reference Smith and Hill19].
Laboratory testing
Stool samples from patients with AFP, GBS, or gastroenteritis were cultured for Campylobacter sp. using standard methods [Reference Fitzgerald, Nachamkin, Versalovic, Carroll, Funke, Jorgensen, Landry and Warnock20]. Isolates were speciated using both phenotypic methods [Reference Barrett, Patton and Morris21] and PCR [Reference Linton, Owen and Stanley22] and were subtyped by Penner serotyping [Reference Penner and Hennessy23], pulsed-field gel electrophoresis (PFGE) [Reference Ribot24], and multilocus sequence typing (MLST) [Reference Dingle25]. Antimicrobial susceptibility testing for azithromycin, ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin, and tetracycline was determined using broth microdilution according to the manufacturer's instructions (Sensititre, Trek Diagnostics, USA).
Serum samples from case-patients and controls were tested for C. jejuni IgM using a validated EIA modified for use with the outbreak strain. The Cochrane–Armitage trend test was used to examine serological results.
Drinking-water samples were tested for Campylobacter sp. and microbial indicators of faecal contamination [26].
All testing was performed at the US Centers for Disease Control and Prevention (CDC).
RESULTS
AFP surveillance and GBS cases
In SLRC, 23 AFP cases were reported with onset from 1 May to 3 July 2011, compared to a baseline of ⩽1 case/year over the previous 5 years. Sufficient information was available to classify 18 cases as GBS using Brighton criteria. An additional eight cases of GBS were identified in Arizona; all met Brighton criteria (levels 1–3). Thus, a total of 26 GBS cases were identified. Paralysis onset ranged from 1 May to 21 July 2011 (Fig. 1 a). The median age was 52 years (range 9–81 years), 21 (81%) patients were male, and 21 (81%) reported antecedent diarrhoea. The median time between onset of diarrhoea and paralysis was 11 days (range 1–30 days). Most patients resided in SLRC (Fig. 2). One patient with pre-existing illness died of complications from GBS.

Fig. 1. (a) Epidemic curve by epidemiological week in 2011 of Guillain–Barré syndome onset in patients by state of residence, (b) reported Campylobacter sp. infections in Yuma County, Arizona, USA, and (c) reported diarrhoeal illnesses in San Luis Río Colorado, Sonora, Mexico, with 25th, 50th, and 75th percentiles of previous 10-year average.

Fig. 2. Approximate locations of residence (circles) of Guillain–Barré Syndrome patients, Yuma County, Arizona, USA, and San Luis Río Colorado, Sonora, Mexico, 2011. Residence locations are jittered to protect confidentiality.
Six of ten GBS patients hospitalized in Arizona (two from Sonora, eight from Arizona) had evidence of antecedent C. jejuni infection (two by stool culture, three by commercial serum IgG antibody assay, one by stool EIA). Of the other four patients hospitalized in Arizona, two had equivocal or negative C. jejuni IgG antibody tests, one had a stool culture taken >2 weeks after onset of GBS that did not yield C. jejuni, and the fourth had no testing for C. jejuni infection. No other potential GBS precipitants (vaccines or other infections) were identified. C. jejuni testing was not available for the 16 patients hospitalized in Mexico. Electrodiagnostic testing was performed in both countries; 14/16 GBS patients tested (88%) had findings consistent with AMAN, one with FS, and one with findings more consistent with AIDP. Two patients hospitalized in Arizona who did not undergo electrodiagnostic testing were diagnosed with FS based upon clinical features.
During extended exploratory interviews with five GBS patients, all denied drinking tap water but reported using 5-gallon (19-litre) water bottles filled at local kiosks. Most also reported consuming fresh chicken, seafood, food from street vendors, and queso fresco (Mexican-style cheese).
Laboratory testing for Campylobacter sp
Stool testing from two GBS patients – one each from Sonora and Arizona – yielded C. jejuni. Both isolates were Penner serotype HS:4 complex, ST132 (clonal complex 508) by MLST, and indistinguishable by PFGE using SmaI and KpnI (PulseNet pattern designation: DBRS16.0070/DBRK02.0084). Both isolates were resistant to tetracycline but not to the other antimicrobial agents tested. The strain with these characteristics is referred to as the outbreak strain. The outbreak strain was isolated from stool specimens from three YC residents with diarrhoea but without GBS.
Surveillance for Campylobacter sp. infection and diarrhoea
YC received reports of 36 cases of culture-confirmed campylobacteriosis from 1 January to 15 July 2011 (Fig. 1 b). Of these, 24 occurred after 1 May, more than twice the number for this period during the previous 2 years. No increase in Campylobacter sp. infections was seen in other Arizona counties. In SLRC, from 8 May to 25 June 2011, reports of acute diarrhoeal illness substantially exceeded the 75th percentile for the preceding 10 years (Fig. 1 c). Of the 76 stool specimens cultured in SLRC, one yielded Salmonella sp.; no other pathogens were identified. Reported Campylobacter sp. infections in YC and diarrhoeal illnesses in SLRC peaked about 2 weeks before the peak in GBS cases.
Case-control investigation
In total, 25 case-patients (18 from Sonora, seven from Arizona) and 74 controls were interviewed. Of the case-patients, 22 had GBS, and three did not but had infection with the outbreak strain.
All seven of the participating Arizona GBS case-patients had visited the SLRC municipality (including the city and nearby agricultural valley) during the exposure window, compared to 9/20 (45%) matched controls (mOR 8·1, 95% CI 1·5–∞) (Table 1). Six of seven Arizona GBS patients had visited the city of SLRC within the municipality. SLRC residents with GBS were not more likely than matched controls to travel to YC during their exposure period (mOR 0·9, 95% CI 0·1–4·6).
Table 1. Selected food and water exposures from Guillain–Barré syndome outbreak case-control investigation, Yuma County, Arizona, USA, and San Luis Río Colorado, Sonora, Mexico, July 2011
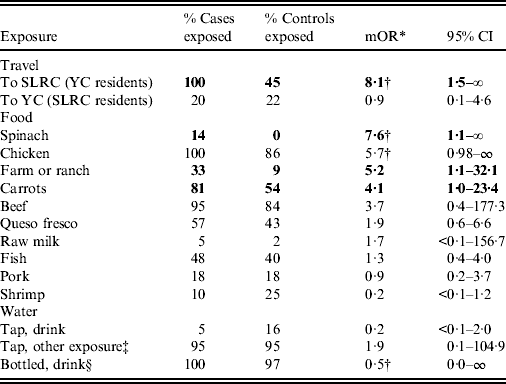
mOR, Matched odds ratio; CI, confidence interval; SLRC, San Luis Río Colorado; YC, Yuma County.
* Matched by age group (2–10 years, 11–25 years, >25 years), sex, ethnicity (Mexican descent or not), and neighbourhood of residence.
† Median unbiased estimate.
‡ Tap water exposure other than drinking (e.g. via ice, brushing teeth, or rinsing produce).
§ Drinking water from refillable, 5-gallon containers.
Because the SLRC area was implicated as the site of exposure, we excluded participants who spent no time there from further analyses of exposures. As a result, two case-patients no longer had matched controls and were also excluded. Table 1 shows associations between illness and selected food, water, and other exposures in this geographically restricted analysis. Exposures to spinach, carrots, and farms or ranches were significantly more common for case-patients than matched controls. There were no significant differences in any other exposures examined. Chicken consumption was reported by all 21 case-patients (100%) with consumption information, compared to 50/56 controls (86%, mOR 5·7, 95% CI 0·98–∞). Nine (43%) case-patients consumed chicken purchased only in the USA and eight (38%) consumed chicken purchased only in Mexico; the production source is unknown. No single chicken variety or brand was commonly reported.
Consumption of water from 5-gallon refillable bottles was universal (100%) for case-patients and nearly as common (97%) for controls, whereas drinking tap water was uncommon (case-patients 5%, controls 16%). Equally high percentages (95%) of both case-patients and controls reported exposure to tap water via ice, brushing teeth, or rinsing produce. Case-patients consumed a significantly higher median number of fruits and vegetables commonly washed and eaten raw than controls (7 vs. 5, P = 0·04). No water vendor, food vendor, or restaurant was reported by more than two case-patients.
Serum antibody testing
Of the case-patients with GBS, 61% (11/18 tested) were seropositive for C. jejuni IgM antibodies, nearly twice the 33% (16/49 tested) of matched controls (mOR 3·5, 95% CI 1·1–13·2). Of the controls, 0 (0%) of nine who resided in YC and did not travel to SLRC were seropositive, two (25%) of eight YC controls who travelled to SLRC were seropositive, and 14 (44%) of 32 controls who resided in SLRC were seropositive (P = 0·01 by trend test).
Excluding controls with positive C. jejuni IgM antibody tests did not meaningfully change results of the case-control investigation. Travel to the SLRC region remained significantly associated with GBS in YC case-patients (mOR 8·6, 95% CI 1·6–∞), as did consumption of spinach and carrots in participants from both countries. However, exposure to farms or ranches was no longer significantly associated.
Environmental investigation
In YC, the Yuma municipal water systems uses both surface water from the Colorado River and groundwater, while the cities of Somerton and San Luis, Arizona use groundwater only. Routine water quality testing in 2011, before and during the outbreak, found no violations and full compliance with residual chlorine requirements. Groundwater in YC contains high levels of iron and manganese, which were removed by an oxidation filtration process.
In the city of SLRC in Sonora, groundwater was the source for all municipal water, with more than 20 borehole wells supplying the city. As the system had no water storage capacity, groundwater was pumped continuously to meet demand. Residents described frequent loss of water pressure. Water was chlorinated at the wellhead using chlorine gas, and polymer sequestration was used to remove iron and manganese; however, sequestration was not completely effective. Residual iron and manganese were oxidized by the chlorine, leading to water discoloration. This discoloration had provoked public complaint, prompting water operators in SLRC to lower chlorine dosage during disinfection. The lowest residual chlorine levels in SLRC were generally in the northern part of the city, which, as the oldest section, also had the oldest water and sewage pipes. Residual chlorine measurements on 1 June 2011 showed markedly low chlorine levels (below the target of 0·2 mg/l) at many sampling points (Fig. 3). Operators increased chlorine levels on 20 June 2011 in response to persistently low levels. Testing of water samples from SLRC wellheads and distribution system in August 2011, about 2 months after the outbreak, detected adenovirus DNA in one of six wells supplying water to northern SLRC. C. jejuni was not detected.

Fig. 3 [colour online]. Maps of San Luis Río Colorado, Sonora, Mexico, with chlorine residual readings (mg/l) by water sector and date. With the exception of the two sectors in the far northwest of the city, water sectors are not independent and water flows freely from one sector to another. Shading is from the lowest chlorine residual (light grey) to the highest residual (black) based on the lowest chlorine residual detected within that sector. The minimum residual chlorine standard in Mexico is 0·2 mg/l; sectors with readings greater than or equal to this level are shaded black. The water sector in the far south of the city does not contain residual chlorine testing sites. Circles represent approximate residence locations of Guillain–Barré syndome patients; locations are jittered to protect confidentiality.
The aquifer underlying YC and SLRC is shallow and exists within permeable sediments [Reference Owen-Joyce27]. Because local precipitation is negligible, groundwater is recharged primarily by water from the bordering Colorado River, which is highly managed through a series of dams and canals [Reference Owen-Joyce27]. The flow of the Colorado River at the northerly international boundary, measured by a stream gauge maintained by the US Geological Survey, peaked on 12 April 2011, at 152 m3/s, more than double the 6-month average of 69 m3/s [28]. YC residents reported minor localized flooding at this time.
DISCUSSION
We describe an outbreak of GBS that included at least 26 cases in a population of ∼400 000 in <3 months – 13–26 times the expected background rate of ∼1–2 cases/100 000 people per year [Reference Sejvar1]. Both clinical and surveillance data indicate that infection with C. jejuni was the precipitant. Six of ten patients hospitalized in Arizona had laboratory evidence of antecedent infection with C. jejuni. More than 80% of patients had diarrhoea in the month before neurological symptoms began. No other potential GBS precipitants were identified despite comprehensive patient testing in those hospitalized in Arizona. Most patients with available information were diagnosed with AMAN or FS, which have strong immunological and epidemiological associations with C. jejuni infection. Furthermore, the C. jejuni serotype isolated from two GBS patients has been associated with FS [Reference Takahashi29].
The timing and magnitude of peaks in acute diarrhoeal illnesses, Campylobacter sp. infections, and GBS cases further implicate C. jejuni as the cause of the GBS outbreak. The increases in acute diarrhoeal illnesses in SLRC and reported campylobacteriosis in YC preceded the peak of the GBS epidemic curve by ∼2 weeks, consistent with the reported time between onsets of diarrhoea and paralysis in GBS patients (median 11 days). A review of stool cultures in SLRC revealed no other likely bacterial cause of the increase in diarrhoeal illness.
Thus, information from Campylobacter sp. and diarrhoeal illness surveillance and the large number of GBS cases indicate that a large, otherwise undetected outbreak of campylobacteriosis probably occurred in SLRC, and to some extent YC, from May to June 2011. The expected incidence of GBS in this region of ∼400 000 people is roughly 4–8 cases/year, or 1–2 cases/3-month period, suggesting that at least 24 or 25 excess GBS cases (of total 26 cases) occurred from May to June 2011. Based on estimates of 0·25–0·65 cases of GBS/1000 cases of C. jejuni infection [Reference Yuki and Hartung4], and assuming 24 outbreak-associated GBS cases, this outbreak could have included 37 000–96 000 cases of C. jejuni infection. However, certain C. jejuni subtypes appear to precipitate GBS more frequently than others [Reference Nachamkin5]. If the outbreak strain led to GBS more often than C. jejuni overall, a smaller number of C. jejuni infections would have occurred. On the other hand, nearly half of controls from SLRC (population 178 380 in 2010) had positive C. jejuni IgM antibody tests, suggesting that recent infection was widespread. Many outbreak-associated infections were probably asymptomatic [Reference Calva9]. That an outbreak of this magnitude was detected primarily via an unprecedented GBS cluster, and secondarily through spillover cases of C. jejuni infection in YC and syndromic surveillance in SLRC, underscores the need for public health surveillance for infection with Campylobacter sp. in Sonora.
The geographical clustering of cases and travel data from the case-control investigation suggest that an exposure within a relatively small area was the source of the C. jejuni outbreak. Travel to SLRC and the surrounding valley was significantly more common in YC GBS case-patients than matched controls, strongly suggesting that exposure to C. jejuni occurred in SLRC.
Food and animal exposures implicated commonly in previous C. jejuni outbreaks were not implicated here. Although consumption of spinach and carrots were both significantly associated with illness, it is unlikely that they were the primary source of C. jejuni exposure. Neither vegetable would explain such a sharply localized outbreak; patients reported purchasing foods from grocery chains serving much wider areas. Although chicken was more commonly consumed by case-patients than controls, the association was not statistically significant, and investigation of varieties and stores revealed no common source of chicken. Exposure to farms or ranches was significantly associated with illness, although only a third of case-patients reported this exposure, also suggesting environmental exposure.
Contaminated municipal water tends to affect people in a relatively restricted area and thus fits this outbreak's distribution. Drinking of tap water was rare (and was more common in controls than cases, although this difference was not statistically significant), but exposure to tap water by brushing teeth or rinsing produce was reported by most participants. Case-patients reported consuming a higher median number of raw fruits and vegetables that are usually rinsed with municipal tap water before eating. These findings suggest that municipal tap-water exposure was common and that case-patients may have had greater exposure than controls.
Because stool testing for C. jejuni was not available in SLRC during the outbreak, and C. jejuni IgM antibody results were not available during the investigation, we instead enrolled as cases all consenting patients with GBS and patients in the USA with C. jejuni isolates matching the outbreak strain. Given the large size of the C. jejuni outbreak, many controls were probably exposed to the pathogen, resulting in outcome misclassification. However, excluding seropositive controls did not meaningfully change the results. A second limitation is the difficulty of implicating water, particularly in a situation with nearly universal, but indirect, exposure and potentially focal contamination. Matching by neighbourhood limited our ability to implicate particular water districts in SLRC. Implicating specific areas might have been easier using a neighbourhood cluster design. However, at the time of the investigation, water was not the only suspect vehicle, and we considered other designs less logistically feasible.
It is not surprising that C. jejuni was not isolated from water samples taken in SLRC. First, C. jejuni is difficult to recover from contaminated water sources even during outbreaks, in part because of small numbers of organisms, low temperatures, osmotic stress, nutrient depletion, and often transient contamination [Reference Koenraad, Rombouts and Notermans30]. Second, in this case, water samples were collected 2 months after the outbreak, after chlorine levels were substantially increased in SLRC. Because C. jejuni is highly sensitive to treatment with chlorine [Reference Jones31], any C. jejuni in the water supply would probably have been eliminated when chlorine levels rose.
Sewage contamination of the water distribution system is a potential source of C. jejuni in drinking water. Parts of the SRLC wastewater system were damaged in a 2010 earthquake, extensive repair work had been ongoing in the northwestern part of the city before the outbreak [32], and low pressure events were frequent, all of which could have allowed for cross-contamination of drinking water.
Because drinking water in SLRC came exclusively from wells, groundwater is another potential source of C. jejuni contamination. The presence of adenovirus DNA at a wellhead in SLRC suggests surface water or sewage intrusion into the groundwater or well. Contaminated groundwater has been the source of outbreaks of campylobacteriosis elsewhere [13, Reference Kuusi33, Reference Stanley, Cunningham and Jones34]. Inadequate disinfection of the water supply at the time of the outbreak could have allowed C. jejuni survival from groundwater to tap. Heavy rainfall events and unusually high runoff from snowmelt have been linked to outbreaks caused by C. jejuni and other enteric pathogens in drinking water [Reference Jones31, Reference Curriero35, Reference Weniger36]. Because the shallow aquifer serving SLRC is hydraulically connected to the Colorado River and is recharged during periods of high flow [Reference Owen-Joyce27], it might be relevant that a marked peak in Colorado River flows with localized flooding occurred 2 weeks before the first cases of diarrhoea [28]. Contaminated surface water could have entered groundwater at that time.
Although the investigation did not indicate the ultimate environmental source of C. jejuni, several possible sources exist in or near SLRC. Birds, a common source of C. jejuni, migrate along the Colorado River and wetlands [Reference Rosenberg37]. Their faeces enter surface water and might seep into groundwater. Surface water might also be contaminated by class B sewage sludge (biosolids) applied to agricultural land in YC; in December 2010, the US Environmental Protection Agency Region IX cited a municipal water district in the state of California for violation of Class B pathogen limits in biosolids applied to YC agricultural lands [38]. Manure is also used extensively as a fertilizer in YC. According to one report, >30 000 tons of chicken manure and nearly 1 million tons of locally produced cattle manure are applied to YC fields annually [Reference Zerkoune39].
This investigation highlights the need for binational, multidisciplinary approaches to cross-border disease outbreaks. Existing health ties, including long-standing Sonora–Arizona collaborative agreements and Mexico–USA cooperation between IHR national focal points, were essential for the coordinated response. The outbreak was felt to meet IHR criteria for a potential Public Health Event of International Concern and thus was reported to the Pan American Health Organization (PAHO) and the World Health Organization by both countries [Reference Kohl17]. Consistent with the integrated response, Mexico and the USA alternately provided PAHO with joint, rather than separate, updates on the outbreak. Both Sonora AFP surveillance data and Arizona campylobacteriosis surveillance data were needed to recognize the cluster and determine its aetiology. The fully integrated case-control and environmental investigations yielded better and more complete data than either country could have gathered independently. A binational laboratory collaboration established laboratory-based Campylobacter sp. surveillance in Mexico, which has since resulted in detection of Campylobacter sp. in stool specimens from patients in SLRC and holds promise for future prevention efforts. The increased flow of information between water managers and experts from both countries has already made the municipal water supply in SLRC safer [Reference Torres40]. The large scope of this outbreak in a region with pressures of an expanding population and shrinking water resources highlights the need for improved drinking- and wastewater treatment management.
APPENDIX
Additional members of the GBS Outbreak Investigation Team
Shoana Anderson, Omar Contreras, Nicole Fitzpatrick, Maureen Fonseca-Ford, Gerardo Gómez, Kevin Joyce, Charles Lacy-Martinez, James Pruckler, Janet Pruckler, Rossanne Philen, Raquel Sabogal, Monica Santovenia, Carmen Vargas, Patricia Griffin.
ACKNOWLEDGMENTS
We thank the many patients, clinicians, and hospitals who assisted with this investigation. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.






