Guillain–Barre syndrome (GBS) is a rare, autoimmune neurologic condition that is preceded by an infection in most cases. Though gastrointestinal illness is the most common, a variety of infectious agents have been reported in association with GBS including SARS-CoV-2. Vaccination is an unusual inciting stimulus for GBS; however, there have been a few recent cases reported following SARS-Cov2 vaccination. We describe a case of variant GBS with facial paralysis and paresthesias as well as sixth nerve palsy occurring after AstraZeneca SARS-CoV2 vaccination. Our review of the current reported cases in the literature suggests that this specific GBS variant may be linked to SARS-CoV-2 adenoviral vaccination.
A 45-year-old man with a history of Crohn’s disease received his first dose AstraZeneca SARS-CoV-2 vaccination. Twelve days later, he noticed weakness in the right side of his face and presented to a community hospital where a noncontrast CT head was normal and he was discharged home with a diagnosis of Bell’s palsy. Over the next 2 days, he developed left facial weakness followed by bilateral severe muscle weakness to the point where he was unable to get out of bed. He had bilateral ascending paresthesias to the knee as well as the left upper limb. At this time, he presented again to hospital where he was admitted for investigations. Over the following days in hospital, he complained of diplopia and was noted to have a right sixth nerve palsy. MRI brain without gadolinium was interpreted as normal. Lumbar puncture showed highly elevated protein (2.0 g/L) with white blood cell count of 14. EMG findings were consistent with demyelinating polyradiculopathy. A diagnosis of GBS was made and treatment was initiated with IVIg. Two months later, he had regained most of the strength in his limbs. Facial paralysis and ocular motility showed only minimal improvement.
GBS is classically characterized by symmetric ascending paralysis and areflexia with a history of antecedent infection in 90% of cases, mostly commonly with Campylobacter jejuni. Reference Koga, Yuki and Hirata1 Several clinical variants exist including Miller–Fisher syndrome, characterized by the triad of ophthalmoplegia, ataxia and areflexia, pharyngeal–cervical–brachial variant and bifacial weakness with paresthesias. Reference Hiew, Ramlan, Viswanathan and Puvanarajah2 Symptoms develop a minimum of 3 and median of 10 days following infection Reference Takahashi, Koga, Yokoyama and Yuki3 and at least in some cases are hypothesized to result from molecular mimicry between bacterial lipopolysaccharides and mammalian nervous system gangliosides. Reference Yuki, Susuki and Koga4 Viral triggers are also reported for GBS including cytomegalovirus, Epstein Barr and Zika virus Reference Jacobs, Rothbarth and van der Meché5,Reference Cao-Lormeau, Blake and Mons6 and now COVID-19. Reference Toscano, Palmerini and Ravaglia7 Whether vaccination is conclusively linked to GBS is matter of some debate, however epidemiological evidence from influenza vaccination campaigns supports a very small risk following vaccine administration.
As mass vaccination campaigns are being carried out worldwide against SARS-CoV-2, reports of GBS are emerging (Table 1). Including the current report, we found 23 cases published in the literature, 21 (91%) of which occurred following AstraZeneca adenoviral vaccination (Table 1). Nineteen of these 21 (90%) cases had bilateral facial palsy and this finding was not present in the two cases following Pfizer messenger RNA (mRNA)-based vaccination. Three cases (13%) had involvement of at least one oculomotor nerve, with two cases of sixth nerve palsy and one of complete ophthalmoplegia. Respiratory symptoms were absent in 65% (15/23), and 17% (4/23) required mechanical ventilation.
Table 1: Guillain–Barre syndrome following SARS-CoV-2 vaccination
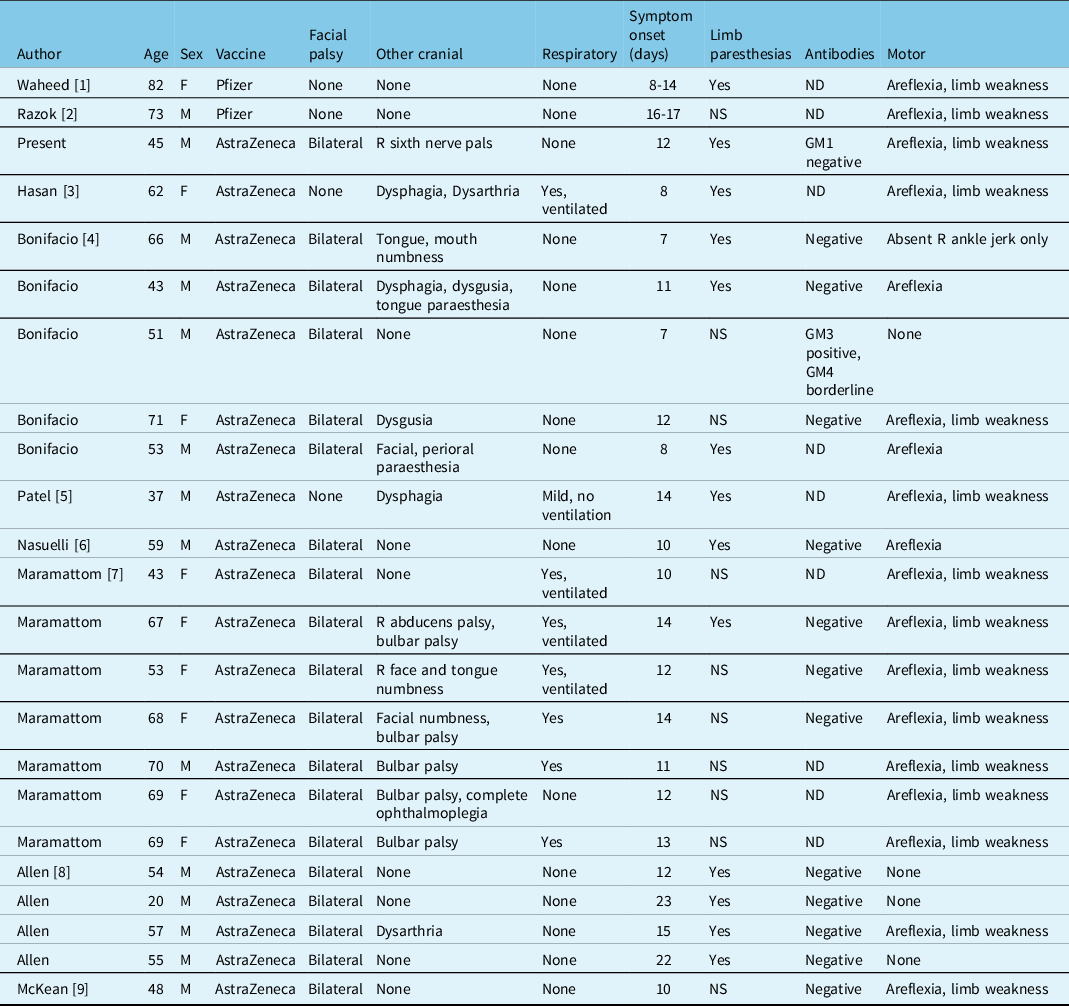
ND: not done; NS: not specified.
Facial palsy is also a frequent finding in GBS following SARS-CoV2-related illness, reported in 42% of cases in a recent systematic review. Reference Aladawi, Elfil and Abu-Esheh8 GBS post-infection with SARS-CoV-2 appears to be more severe than when it follows vaccination, with 33% of cases requiring mechanical ventilation and 6% proving fatal. The pathophysiology of post-infectious and post-vaccination GBS in SARS-CoV-2 is unknown; however, if molecular mimicry between the virus and mammalian gangliosides is present it would have to be within the spike protein as this is the common target of all currently available vaccines.
Notably, a causative link is not present in all cases of GBS following COVID-19 or SARS-CoV-2 vaccination. One case of GBS occurred in the vaccine arm and one in the control arm in trials for the Johnson & Johnson adenoviral vaccine. Reference Márquez Loza, Holroyd, Johnson, Pilgrim and Amato9 A case of GBS 1 day following mRNA Pfizer SARS-CoV-2 vaccination is also reported, which is outside of the window of reported GBS cases being too rapid in onset. Large epidemiologic studies will be required to determine whether these reported cases are causatively linked.
Patients presenting with bilateral facial palsy should be asked about the recent SARS-CoV-2 vaccination and also be tested for serologic evidence of infection so that the diagnosis of GBS may be considered. Cases without other classic features of this syndrome may otherwise be missed, as initially occurred in the current case, or facial palsy may be the only complaint reported. Correct diagnosis is essential so that patients are monitored for signs of respiratory compromise. In addition, reporting of all suspected cases will help to determine whether there is a true causative link between SARS-CoV-2 vaccination and GBS.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.492
Funding
There are no funding sources to disclose.
Disclosures
The authors have no conflicts of interest.
Statement of Authorship
Both authors contributed equally to data collection, writing, and editing of this manuscript.



