The majority of trigeminal autonomic cephalalgias (TACs) are primary headache syndromes but secondary etiologies should be excluded. In the presence of TACs, imaging is recommended in order to rule out secondary causes which include structural, vascular, demyelinating, and inflammatory lesions.Reference Cao, Yang, Dong, Huang, Cao and Yu1,Reference Goadsby and Lipton2
We describe the case of a 52-year-old man who presented to the emergency room after being awakened by a new-onset paroxysmal episode of intense pain in the left periorbital area lasting 5–10 min accompanied by ipsilateral eye tearing, rhinorrhea, and conjunctival injection. Left paranasal paresthesia extending into the maxillary branch was reported. A second identical episode followed shortly before being evaluated by the neurology team. The patient had no past medical history and denied taking any medication, supplements, or illicit drugs.
The physical examination revealed an afebrile patient, appearing well interictally but in severe pain during the paroxysmal headache. At first assessment, minutes after his second headache, left-sided conjunctival injection and rhinorrhea were observed. A complete neurological examination revealed a slight hypoesthesia in the V2 branch of the left trigeminal nerve.
Initial laboratory workup was negative.
The clinical presentation was suggestive of TAC, and the short duration of the episodes was compatible with a short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) or paroxysmal hemicrania (PH) as the two conditions overlap with regard to their attack duration criterion. Criteria that were not filled include the minimum requirement of 20 attacks, the response to indomethacin (for PH), and the exclusion of a secondary etiology.
Indomethacin was introduced as a therapeutic and diagnostic trial.Reference Weng, Cohen, Schankin and Goadsby3
No further episodes were noted upon indomethacin introduction, supporting the diagnosis of PH, but the baseline frequency of attacks was not clearly determined due to a short observation period before the treatment.
A computed tomography (CT) scan of the head (Figure 1) and subsequent brain magnetic resonance imaging (MRI; Figure 2) demonstrated a hemorrhage of the fascicular and proximal cisternal segments of the left trigeminal nerve with minimal mass effect. CT and MR angiography showed no underlying vascular lesion or suspicious enhancement.

Figure 1: Brain computerized tomography demonstrating hyperdensity following the left trigeminal nerve at its cisternal segment.
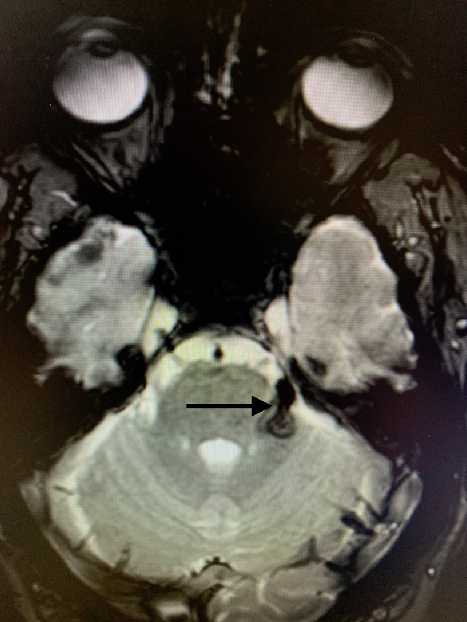
Figure 2: Brain magnetic resonance imaging demonstrating susceptibility-weighted hypointensity following the emergence of the left trigeminal nerve.
The patient was weaned off indomethacin over a 4-week period and sent home with close follow-up. Six weeks after discharge, the patient described the reemergence of multiple episodes of intense stabbing periorbital paroxysmal headache lasting 1–2 s, without autonomic features, recurring every hour which subsequently responded to reintroduction of indomethacin. Upon subsequent follow-up, 3 months later, the patient remained asymptomatic and was successfully weaned off indomethacin with no residual deficits. The patient’s clinical presentation corresponded to all International Classification of Headache Disorders (ICHD-3) diagnostic criteria for a trigeminal neuralgia attributed to cause other than the lack of precipitation by innocuous stimuli within the affected trigeminal distribution.
A follow-up MRI showed involution of the localized bleeding following the trigeminal nerve, with no postcontrast enhancement or underlying mass.
The slight edema in the original MRI was attributed to recent bleeding which was in accordance with the hypersignal documented on the original head CT scan. The initial imaging differential diagnosis included an atypical cavernous lesion, thrombosed vascular lesion, perineural metastasis, or schwannoma. All appeared unlikely, considering the complete normalization upon subsequent imaging.
The TACs are a subgroup of primary headache disorders characterized by severe unilateral pain in the trigeminal nerve distribution associated with ipsilateral paroxysmal facial autonomic symptoms. They include cluster headache, PH, hemicrania continua, and short-lasting unilateral neuralgiform headache with autonomic features which are distinguished by the duration and frequency of episodes and also in their response to specific treatments.
A review published in 2007 described 31 cases of TAC and TAC-like disorders attributable to structural lesions. Of those, 21 filed the ICDH diagnostic criteria for TAC, whereas 10 had atypical features including duration, frequency, continuous headache and absence of autonomic symptoms and/or bilateral symptomatology. Of the structural lesions, 10 were vascular in origin, 18 were tumoral including 11 pituitary tumors, 1 was infectious, 1 was related to a foreign object, and 1 was attributed to a causal mucocele.Reference Favier, Van Vliet and Roon4
In addition, several case reports describe the occurrence of secondary etiologies of TAC attributable to a vast array of central nervous system insults. Although literature describes that 15% of TAC may be associated with hypoesthesia in the V1 or V2 trigeminal branch, such neurological deficits increase the likelihood of a secondary etiology.Reference Cohen, Matharu and Goadsby5 Other red flags have been identified in literature, but their absence should not substitute the need for neuroimaging. The presence of structural intracranial pathology has been shown to account for headache in half of typical TAC presentations fulfilling ICHD criteria. Upon review, half of those cases exhibited certain atypical features. Interestingly, one third of patients with secondary TAC exhibited an episodic pattern of headache, reinforcing the fact that no clinical characteristic should preclude imaging indication.Reference Chowdhury6
TACs can be treated by stimulation of the occipital nerve (part of the trigeminovascular system), the sphenopalatine ganglion (cranial autonomic system), or the posterior thalamus, thus suggesting a pathophysiological link between the trigeminal autonomic reflex, the hypothalamic–trigeminal nucleus connections, and the hypothalamic autonomic axis.Reference Leone and Bussone7,Reference Goadsby8
Our case depicts a secondary cause of headache presenting with a trigeminoautonomic phenotype due to direct irritation of the trigeminal nerve, hypothetically activating this trigeminal autonomic loop. The duration and frequency were suggestive of SUNCT, the impressive response to indomethacin was compatible with PH and then symptoms evolved into a trigeminal neuralgia phenotype. The overlap observed between SUNCT, PH, and trigeminal neuralgia could potentially be explained by the progressive resorption of the hemorrhage, decreasing the trigeminoautonomic loop activation while still irritating the underlying trigeminal nerve.
This case illustrates the critical importance of imaging any patient presenting with new-onset TAC. It appears noteworthy that TAC attributable to an underlying structural lesion can present as a typical TAC that may fulfill all ICDH diagnostic criteria, justifying the low imaging threshold. Secondary causes of TAC attributed to structural lesions are important to recognize and have been shown to improve after the treatment of the underlying lesion, suggesting the causal role of the lesion.Reference Favier, Van Vliet and Roon4 To our knowledge, no other cases presenting and evolving as such have been published and documented in medical literature.Reference Wei, Ong and Goadsby9
Acknowledgements
We would like to acknowledge and thank the patient described anonymously in this case report for allowing us to describe his case and use his images.
Disclosures
None of the authors have financial or proprietary interests in any of the material or method mentioned.
Statement of Authorship
ML: Evaluated the patient, posed the diagnosis, and obtained patient consent; reviewed literature and wrote the case report; study concept and acquisition of data. CB: Revision of manuscript. FM: Revision of manuscript and supervision of patient care and follow-up.




