Individuals with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) risk developing opportunistic infections including Mycobacterium tuberculosis complex (Mtbc) and organisms belonging to the Mycobacterium avium complex (MAC). Disseminated infection of a newer member, M. genavense, more commonly observed in birds, has also been reported Reference Hirschel, Chang and Mach1–Reference Pechère, Opravil and Wald3 with two cases presenting as a solitary brain mass. Reference Berman, Kim, Haghighat, Mulligan, Fierer and Wyle4,Reference Toussi, Goodarzi, Kulubya, Lee and Waldau5 Unlike other organisms of the genus, M. genavense does not grow on solid conventional media, taking up to 6–12 weeks in liquid media, and requiring additional molecular techniques for timely identification. Reference Realini, Van Der Stuyft, De Ridder, Hirschel and Portaels6,Reference Realini, De Ridder, Hirschel and Portaels7 We present an additional case of M. genavense solitary central nervous system (CNS) infection with evidence for immune reconstitution inflammatory syndrome (IRIS) in a patient with HIV/AIDS.
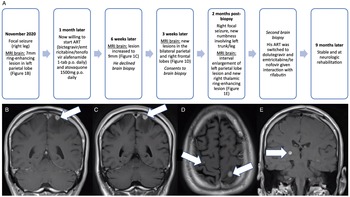
A 54-year-old man presented to the emergency department in November 2020 with a focal seizure involving his right leg. Figure 1A demonstrates the patient’s presentation timeline. He was diagnosed with HIV/AIDS in 2014 and was not taking antiretroviral therapy (ART) for the last 5 years. His medical history included previous opioid use. He was hemodynamically stable and afebrile, without constitutional symptoms, and unremarkable basic blood work (WBC 5.14). MRI brain identified a 7-mm ring-enhancing lesion in the left parietal cortex (Figure 1B). Neurologic examination was normal and he started levetiracetam 750 mg p.o. BID. Lumbar puncture demonstrated normal leukocyte count (WBC 4, reference range 0–5 × 106/L; 100% lymphocytes), mildly elevated protein of 0.50 (reference range 0.15–0.45 g/L), normal glucose of 3.3 (reference range 2.5–4.2 mmol/L), and negative bacterial/viral/fungal/parasitic studies and cytology, including negative acid-fast bacilli (AFB). Full-body CT ruled out malignancy, and infectious workup ruled out infective endocarditis and bacteremia. CD4+ count was 166 (reference range 500–1200 cells/mm3), with viral load of 26,196 copies/mL. Initially suspecting bacterial abscess, he was empirically treated with ceftriaxone 2 g IV daily and metronidazole 500 mg IV q8h. MRI brain 10 days later demonstrated a reduction in mass size to 6 mm. Given atypical neuroimaging findings and negative serology, he was not started on Toxoplasmosis treatment. At 3 weeks of treatment, he developed rash and antibiotics were switched to meropenem 2 g IV q8h.

Figure 1: Patient presentation timeline and pertinent neuroimaging throughout the clinical presentation. (A) Timeline of patient’s presentation and progression of CNS infection. (B) Coronal post-gadolinium image at presentation demonstrating a 7-mm ring-enhancing lesion in the left parietal lobe closely attached to the dura at the vertex with vasogenic edema. (C) Coronal post-gadolinium image identifying increased size of the left parietal lobe lesion to 9 mm with associated vasogenic edema 6 weeks after initial presentation. (D) Axial post-gadolinium image demonstrating slight increase in size of the left parietal lobe lesion and new lesions in the right posterior frontal lobe measuring 3-mm 9 weeks after initial presentation. (E) Coronal post-gadolinium image demonstrating a 6-mm ring-enhancing right thalamic lesion in May 2021, 2 months post-first biopsy. Abbreviations: antiretroviral therapy (ART), central nervous system (CNS).
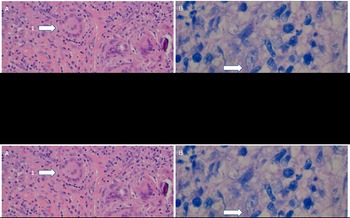
Six weeks after initial presentation, MRI brain demonstrated progression (Figure 1C). Further progression three subsequent weeks later on MRI brain was identified (Figure 1D), and he consented to biopsy. Surgical pathology/microbiology revealed AFB with Ziehl–Neelsen stain (Figure 2A-B). He started treatment for presumed M. tuberculosis with isoniazid 300 mg p.o. daily, rifabutin 300 mg p.o. daily, pyrazinamide 1500 mg p.o. daily, levofloxacin 750 mg p.o. daily, and prednisone 40 mg (4 weeks with taper). PCR testing was negative for Mtbc and MAC. Direct PCR of biopsy material at 3 weeks post-craniotomy resulted in a 16S rRNA gene product (567 bp) with 99% match and hsp65 product (387 bp) with 100% match to M. genavense type strain 2289. Upon identification of M. genavense, pyrazinamide and isoniazid were discontinued and he continued levofloxacin 750 mg p.o. q2days, azithromycin 500 mg p.o. daily, and rifabutin 300 mg p.o. daily. Ethambutol was not added at this time due to new renal dysfunction (creatinine 150 μmol/L, reference range 60–116 μmol/L; urea 11.7 mmol/L, reference range 3.0–7.0 mmol/L; estGFR 42/min/173 m2, reference range >60/min/173 m2).

Figure 2: Molecular characteristics of the left parietal lobe M. genavense lesion. (A) Hematoxylin and eosin stain demonstrating granulomatous inflammation with Langerhans giant cells (arrow 1) admixed with lymphocytes in a collagenous stroma (arrow 2). (B) Ziehl–Neelsen stain for acid-fast bacilli, which appear as red rods scattered throughout. The arrow points to a cluster. (C) Histopathology of the second biopsy material with an arrow identifying an area of caseating necrosis.
There was further clinical and neuroimaging progression 2 months post-biopsy (Figure 1A, 1E). Given improved renal function, ethambutol 900 mg p.o. daily was added to the M. genavense regimen. To rule out another infectious agent, he underwent a second craniotomy which found thick, indurated intra- and extra-axial matted material. Pathology identified necrotizing granulomatous inflammation, but no AFB or other infectious agents (Figure 2C). He was restarted on prednisone 50 mg p.o. daily with slow taper for presumed IRIS. MRIs at 1 and 3 months after the second craniotomy showed decreasing edema and stable right thalamic lesion. He remains neurologically stable with undetectable viral load 9 months after initial presentation.
Disseminated M. genavense infection can occur in patients with HIV/AIDS with CD4+ counts <100, with median survival of 6.3 months. Reference Pechère, Opravil and Wald3 It was first described in 1990 in a patient with AIDS who succumbed to his infection. No organism could be cultured and nucleic acid testing indicated that the organism was not M. avium. Reference Hirschel, Chang and Mach1 Böttger et al. (1992) later grew AFB in liquid medium and DNA amplification and sequence analysis identified the organism as a new member of the genus Mycobacterium Reference Böttger, Teske, Kirschner and et. al.2 in patients with disseminated M. genavense infection. These first two cases Reference Hirschel, Chang and Mach1,Reference Böttger, Teske, Kirschner and et. al.2 did not involve the CNS. Two subsequent biopsy-confirmed cases of M. genavense presenting strictly as a solitary brain abscess were later reported. Reference Berman, Kim, Haghighat, Mulligan, Fierer and Wyle4,Reference Toussi, Goodarzi, Kulubya, Lee and Waldau5 To our knowledge, this is the third reported case of a patient with HIV/AIDS presenting with isolated CNS M. genavense infection. Like previous reports of M. genavense, Reference Berman, Kim, Haghighat, Mulligan, Fierer and Wyle4 our histopathology demonstrated lymphohistocytic inflammatory infiltrates, organized in granulomata. Interestingly, our patient had a CD4+ count >100, unlike most cases of disseminated Reference Böttger, Teske, Kirschner and et. al.2 and solitary Reference Berman, Kim, Haghighat, Mulligan, Fierer and Wyle4 M. genavense infection in HIV-positive patients.
Optimal therapy for M. genavense is not well established, with previous studies utilizing macrolide antibiotics, rifabutin, and ethambutol. One report in 1999 suggested that isoniazid has limited efficacy and ethambutol has uncertain susceptibility against M. genavense. Reference Thomsen, Dragsted, Bauer, Fuursted and Lundgren8 Knowing the species of Mycobacterium, therefore, was important in adjusting the treatment regimen. In our case, the lesions continued to evolve, with likely multifactorial causes including (1) initial regimen against M. genavense did not include ethambutol due to temporary renal impairment, (2) initial use of isoniazid for presumed M. tuberculosis may have been ineffective against M. genavense, (3) paradoxical worsening secondary to IRIS, and/or (4) delayed brain biopsy given patient hesitancy which may have affected treatment. Results from the second brain biopsy may further support the diagnosis of IRIS given the abundance of inflammatory cells in the absence of Ziehl–Nielsen detectable bacilli; these findings may represent a florid inflammatory response to bacteria that were eliminated through successful treatment. In this case, IRIS may have developed secondary to opportunistic M. genavense CNS infection and concomitant ART. The present case demonstrates the fastidious and poorly understood nature of M. genavense, contributing to its diagnostic challenge. Mycobacterium genavense should be considered early in immunocompromised patients presenting with brain abscess(es) and AFB-positive staining after Mtbc and MAC have been ruled out; in such cases, molecular testing must be requested.
Disclosures
The authors declare that there is no conflict of interest. Dr Ostrowski has received CIHR and NIH research operating grants, and participates on a Data Safety Monitoring Board for a COVID-19 trial with anticoagulants not affiliated with this case.
Statement of Authorship
AMK, SK, and ASB participated in the drafting of this manuscript. JS, JVK, DGM, MO, and YC participated in manuscript revisions. DGM provided histopathology for the manuscript.