CLINICIAN'S CAPSULE
What is known about the topic?
There is a wide variety of point-of-care ultrasound (POCUS) scanning protocols for traumatic pneumothoraces in the literature.
What did this study ask?
What is the optimal area that needs to be scanned with POCUS to diagnose a pneumothorax as seen on computed tomography?
What did this study find?
The optimal areas on POCUS are the parasternal border and mid-clavicular line from the inferior aspect of the clavicle to the physiologic lung point (liver on the right, heart on the left)
Why does this study matter to clinicians?
This protocol has the potential to standardize practice and reduce time to diagnosis of a traumatic pneumothoraces.
INTRODUCTION
Point-of-care ultrasound (POCUS) assists in the early recognition of a pneumothorax in trauma. A recent meta-analyses found that POCUS had a sensitivity of 53–100% and a specificity of 93–100% for a pneumothorax.Reference Ebrahimi, Yousefifard and Kazemi1 POCUS scanning protocols varied in the 28 analyzed studies. This may partly explain the wide range in sensitivity. Protocols differed in the number of areas of the chest that were scanned with POCUS and the locations where the ultrasound probe was placed. In the vast majority of these studies, no basis for the scanning protocol was provided.
As computed tomography (CT) is the gold standard for detection of pneumothoraces, probe placement in a scanning protocol should be based on the distribution of air as seen on CT scan. As the use of POCUS increases, we must ensure its use is efficient and accurate, all the while understanding its possible limitations. With such a wide variety of scanning protocols, it is important that any protocol includes areas where most pneumothoraces are likely to be found.
Our study aims to delineate this distribution of pneumothoraces within the chest cavity and correlate it with external anatomical and internal sonographic landmarks. Our results will offer an evidence-based rationale for an area that should be included in any scanning protocol for pneumothorax in supine trauma patients.
METHODS
This study was performed at the Health Sciences North (HSN) emergency department (ED) in Sudbury, Ontario. The HSN ED is the sole ED for Sudbury that has a population of 160,000 and an annual volume of 76,000 patients. HSN serves as the trauma center for Northeastern Ontario. Ethics approval was through the HSN ethics review board.
Study patients were selected following a retrospective review of the hospital's trauma registry from November 2006 to August 2016. The registry was searched for patients with a diagnosis of pneumothorax. Adult patients were included if a pneumothorax was diagnosed on CT scan. Patients with bilateral pneumothoraces had each hemithorax evaluated and tabulated separately. Based on this chart review, 304 traumatic pneumothoraces were identified, and their CT scans were assessed for eligibility. Patients were excluded if they did not have an identifiable pneumothorax on CT or if they underwent a tube thoracostomy before CT. Penetrating and blunt trauma patients were eligible.
CT images of the 304 patients were analyzed. The location (or zone) of the pneumothorax was recorded based on predefined anatomic landmarks (Figure 1). We used a novel definition of zones as we required a combination of internal and external anatomic landmarks, which would be identifiable on CT/US (ultrasound) as well as on the patient's surface anatomy (Figure 1). If a pneumothorax was found within the borders of the zone, this zone was considered positive for containing a pneumothorax.
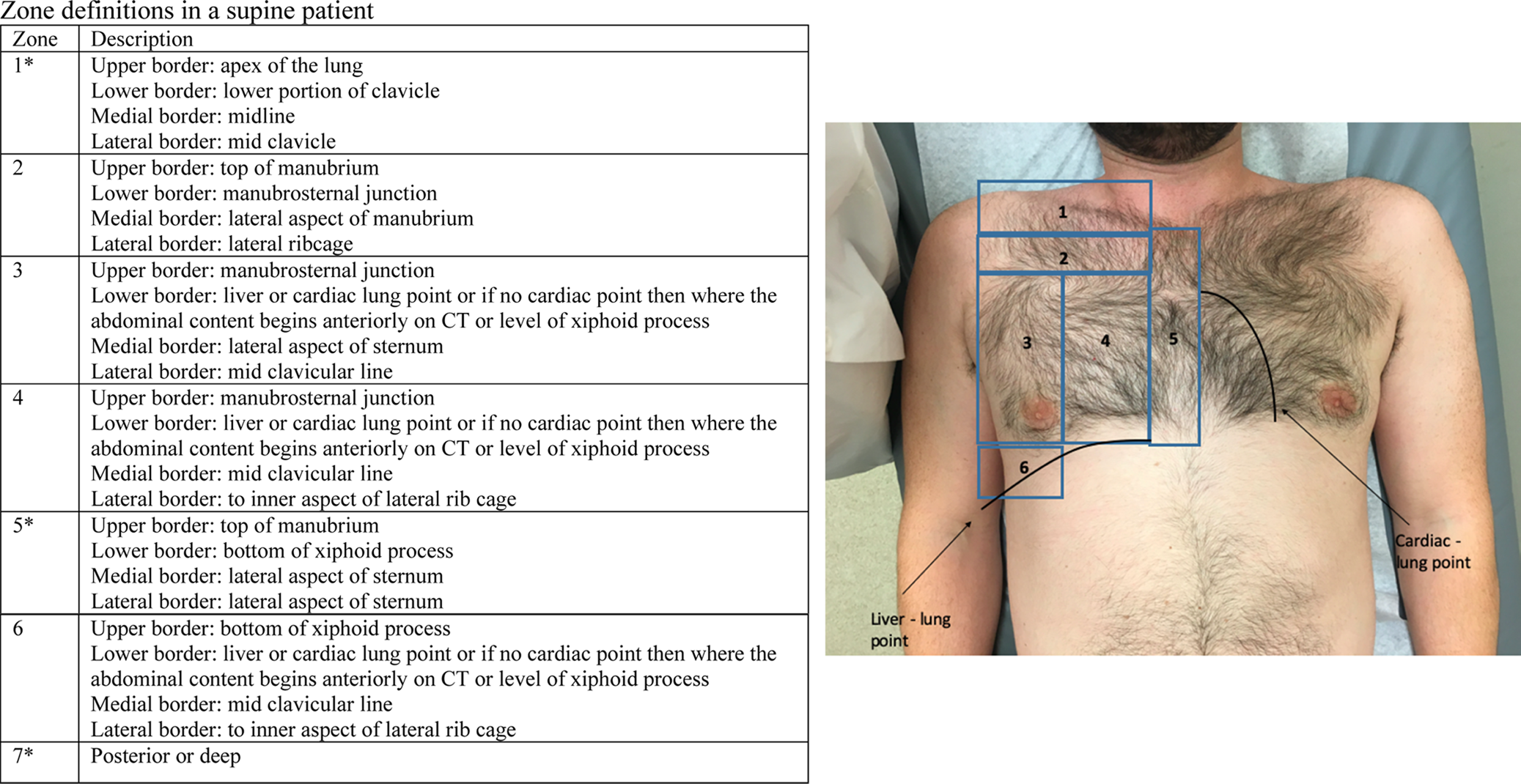
Figure 1. The zones of the pneumothorax, based on predefined anatomic landmarks in a supine patient.
Data collection was completed by a single researcher following a training session with the supervised review of 20 charts to ensure proper identification of the pneumothorax location. A second investigator reviewed 20% of the images to assess inter-rater reliability, measured using the kappa statistic. The study was conducted according to STARD (Standards for Reporting of Diagnostic Accuracy Studies) reporting guidelines.
RESULTS
Data collected from 2006 to 2016 yielded 170 traumatic pneumothoraces on CT with an average age of 44.2 and 77.8% male (Supplemental Figure 1; Supplemental Table 1). The kappa for data extraction was 0.88. Bilateral pneumothoraces were present in 19.4% of patients, leading to a total sample size of 203 (Supplemental Figure 1).
Of the seven defined anatomical zones, zone 3 had the highest number of pneumothoraces (85.7%). Zone 4 had the second highest number of pneumothoraces (80.8%). Zones 1 and 2 had 69 (34%) and 80 (39.4%) pneumothoraces, respectively, representing the two zones least likely to contain a pneumothorax (Supplemental Table 2).
Supplemental Table 3 shows the theoretical sensitivities of the zones accessible by an ultrasound probe in a supine patient. Combining the zones increased the sensitivity of detecting a pneumothorax. The most sensitive combination of zones for the detection of a pneumothorax was zone 2, 3, and 4, with a theoretical sensitivity of 91.6% (95% confidence interval, 86.9–95). Of the 18 pneumothoraces that would be missed, 17 were in “theoretically inaccessible” zones (3 in zone 1; 12 in zone 7; 2 combination of zone 1 and 7). All of these pneumothoraces were described as “tiny” or “miniscule” on the radiology reports. Of the 18 missed pneumothoraces, 3 had chest tubes inserted during their course in hospital.
DISCUSSION
In our study, we mapped the majority of pneumothoraces to an area defined by the inferior border of the clavicle at the para-sternal border down to the liver or cardiac lung points and then repeated again at the midclavicular line (zones 2, 3, 4; Figure 1). When using this as a scanning area, we obtained a theoretical sensitivity of 91.6%; this is comparable to previously reported sensitivities of POCUS for traumatic pneumothorax.Reference Helland, Gaspari and Licciardo2–Reference Volpicelli, Elbarbary and Blaivas4 There are many scanning protocols that vary in their inclusion of these areas.
The Hellard protocol does not include scanning zones 2 or 6 (Figure 1 and Supplemental Figure 2).Reference Helland, Gaspari and Licciardo2 This would theoretically lead to an additional three pneumothoraces being missed. The protocol by Blaivas et al.Reference Blaivas, Lyon and Duggal3 includes zones 2, 4, and 6, which may miss an additional eight pneumothoraces isolated to zone 3 (Supplemental Figure 2). The BLUE protocol would scan zones 2 and 3, and if the patient were turned sufficiently, zone 7; this would miss 8 pneumothoraces in zone 4 but potentially diagnose some of the 12 pneumothoraces that were in zone 7. The BLUE protocol suggests minimal movement, so it is unlikely to pick up all 12 pneumothoraces. The Volpicelli protocol requires scanning the entire chest but does include zones 2, 3, and 4. This is not an exhaustive list of the many scanning protocols. What is important to note is that, whatever technique you use, it should include the anatomical areas defined by zones 2, 3, and 4.
Some areas of the chest cavity are inaccessible to the ultrasound probe, notably zones 1, 5, and 7. A total of 18 pneumothorax were found in isolation in these areas. Clinically, these were reported as “tiny” or “miniscule” on the CT reports. Only three of these required chest tube insertion. Two required chest tube insertion on days 2 and 10, respectively, with the third requiring insertion on the same day due to the need for intubation to undergo a splenectomy. It should be noted that pneumothoraces that are not detected on initial ultrasound due to inaccessible location could still be clinically significant in the future.
LIMITATIONS
This study is limited by its retrospective nature; we did not have the opportunity to scan these patients with ultrasound and compare with the CT findings. Therefore, the diagnostic accuracy is theoretical; however, it is comparable to previously reported literature.Reference Ebrahimi, Yousefifard and Kazemi1–Reference Staub, Biscaro, Kaszubowski and Maurici5 Patients were recruited from the institutional trauma registry; therefore, cases that were not enrolled in this registry would be missed. There is a potential that patients who were more severely ill were not included in the registry. However, all CT scans done in the ED related to trauma are routinely reviewed for eligible patients for the registry; thus, missed cases are rare.
A CT scan produces a static image that represents a “snapshot in time”. In contrast, ultrasound scanning is dynamic, with motion inside the thorax being visible on the screen. The location of a pneumothorax changes with respiration. However, based on the collective experience of the authors, that change is only slight. Therefore, despite this difference between CT and ultrasound, we believe that it is valid to use CT to help define the areas where a pneumothorax could be found.
CONCLUSION
This study suggests any scanning protocol used should include an area from the inferior border of the clavicle at the parasternal border down to the liver or cardiac lung points and then the mid clavicular line down to the liver or cardiac lung points.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2020.21.
Acknowledgments
Dr. Socransky was responsible for study conception and oversight in addition to data analysis. Dr. Bignucolo was responsible for data extraction and manuscript preparation he acts as guarantor of the article, taking responsibility for the integrity of the work as a whole, from inception to published article. Dr. Acton assisted with data extraction. Dr. Ohle provided methodological support and aided in manuscript preparation.
Competing interests
No conflict of interest for any authors.



