The taste of the amino acid glutamate, alone and in combination with the ribonucleotide inosine 5′-monophosphate (IMP-5), is thought to represent the taste of dietary protein. Glutamate is found in nearly all protein-containing foods (for example, cheese, fish, meat, poultry, eggs) and many vegetables (for example, tomatoes, mushrooms, cabbage, maize, green asparagus) and IMP-5 is found primarily in meat and some types of fish such as tuna(Reference Ninomiya1). The taste of glutamate (administered orally in the form of monosodium glutamate (MSG)) has been described as ‘Umami’ by the Japanese (meaning savoury deliciousness) or as ‘meaty, broth-like, savoury’ by Westerners(Reference Yamaguchi and Ninomiya2). The Umami taste is recognised and coded as a unique quality by the taste system and is therefore considered the fifth basic taste(Reference Rolls3, Reference Chaudhari, Landin and Roper4). A special quality of glutamate is its ability to enhance several specific flavour characteristics of food(Reference Bellisle, Tournier and Louis-Sylvestre5–Reference Prescott10). Moreover, behavioural and electrophysiological studies in human subjects and non-humans have found that glutamate interacts synergistically with certain other stimuli such as IMP-5 to accentuate the intensity of the Umami taste even when only a low concentration of free glutamate is naturally present in food(Reference Kurihara and Kashiwayanagi11, Reference Delay, Beaver, Wagner, Stapleton, Harbaugh, Catron and Roper12).
Evidence for glutamate and IMP-5 representing the taste of protein, however, remains scarce. Studies in rats, non-primates and human subjects have demonstrated that a preference for dietary protein (or liking of) is almost certainly related, in part, to the way the body senses and tastes MSG ± IMP-5 and on the body's protein requirements and stores at the time(Reference Smriga and Torii13–Reference Laska and Hernandez Salazar19). Recent studies in α-gustducin and rod α-transducin knock-out mice have shown that Umami taste detection involves the activation of these two G-protein-coupled receptors which are located in specialised epithelial cells of the anterior taste bud(Reference He, Yasumatsu, Varadarajan, Yamada, Lem, Ninomiya, Margolskee and Damak14, Reference Ruiz, Wray, Delay, Margolskee and Kinnamon15). In addition, taste buds at the back of the tongue respond to Umami compounds independently of the G-protein subunits(Reference He, Yasumatsu, Varadarajan, Yamada, Lem, Ninomiya, Margolskee and Damak14). Given that a specialised tasting system exists to recognise the Umami taste, it is only natural to speculate that this distinctive taste functions as a signal to regulate protein intake. Supporting this notion is the finding that individuals with low nutritional or protein status preferred higher concentrations of MSG in solution than those who were better nourished(Reference Murphy, Kawamura and Kare20).
The aim of the present study was to determine individuals' taste threshold for MSG (alone and in combination with IMP-5) and to examine if this threshold was related to an increase in sensory properties (including pleasantness of taste) and/or to one's preference for dietary protein over carbohydrate and fat.
Subjects and methods
Subjects
Sixty subjects aged 19–63 years were recruited from responders to advertisements in a local newspaper and around the Maastricht University. Inclusion criteria were to be healthy, to be a non-smoker, on no medication (except the contraceptive pill), not known to be allergic to MSG or other foods, not dietary restrained (assessed using the Three Factor Eating Questionnaire(Reference Stunkard and Messick21)), low to moderate alcohol use (i.e. ≤ two standard drinks per d for no more than 5 d per week) and a BMI between 20 and 30 kg/m2 and weight stable ( < 3 % change over the 3 months before screening). Fat and fat-free mass was determined for each subject from the measurement of total body water using 2H dilution and the assumption that total body water occupies an approximate average of 73·2 % of the fat free mass(Reference Westerterp, Wouters and van Marken Lichtenbelt22, Reference Westerterp23). Thirty-six women (age 31 (sd 15) years; BMI 23·5 (sd 2·5) kg/m2; fat mass 20·3 (sd 6·9) kg; fat-free mass 46·1 (sd 5·4) kg; dietary restraint score 6·3 (sd 2·9)) and twenty-four men (age 38 (sd 15) years; BMI 25·9 (sd 2·4) kg/m2; fat mass 21·3 (sd 12·0) kg; fat-free mass 63·0 (sd 7·4) kg; dietary restraint score 8·5 (sd 3·8)) were enrolled in the study that was approved by the Medical Ethics Committee of Maastricht University. All subjects gave written informed consent to participate.
Study design
On two separate experimental sessions, at least 1 week apart, each subject participated in a series of single-blinded ‘triangle taste tests’ to determine the taste threshold of glutamate (i.e. the lowest concentration correctly recognised) in a clear soup when MSG alone, or in combination with IMP-5(Reference Kamphuis, Saris and Westerterp-Plantenga24), is added. On each day, subjects completed questionnaires regarding the sensory properties of the soup and their ‘liking’ and ‘eating frequency’ of high-carbohydrate, -fat and -protein food items.
Preparation of soup, monosodium glutamate and inosine 5′-monophosphate
The soup stock was prepared with 20 g Vectra® vegetable boullion (Natudis B.V., 3480 AJ, Harderwijk, The Netherlands) to 1 litre of water and heated to about 65°C. Herbs were strained from the stock so that only a clear broth was used for the taste tests. The manufacturer's nutritional analysis of the soup documented it as containing no glutamate. MSG and IMP-5 were purchased as salts from Ajinomoto Foods Germany GmbH (Hamburg, Germany) and they were thoroughly mixed into the soup a few minutes in advance of tasting. For triangle taste tests determining the recognition threshold of MSG alone, the concentration of MSG added to the soup ranged 0·1–0·8 % (weight of MSG/weight soup). When determining the recognition threshold of MSG in combination with IMP-5, a constant concentration of 0·25 % (w/w) IMP-5 was added to the MSG soup solution. All concentrations of MSG and IMP-5 were within the ranges that are typically added to commercial food and were similar to levels of naturally occurring glutamate found in traditional dishes(Reference Bellisle25, Reference Homsey26).
Triangle taste tests and characterisation of tasters of monosodium glutamate alone and in combination with inosine 5′-monophosphate
Using the ‘triangle tasting method’(Reference Kamphuis, Saris and Westerterp-Plantenga24) the taste threshold of MSG ± IMP-5 for individuals was determined. Each ‘triangle taste test’ involved the presentation of ten rows of triplicate cups with 8 ml soup (total of thirty cups). Within each triplicate, either one or two cups contained soup with added MSG ± IMP-5. The remaining cups contained soup with no additive(s). Subjects were instructed to taste (but not swallow) half the volume of each soup cup and they were allowed to re-taste each sample if necessary. They were asked to ‘identify the soup with the added ingredient(s)’ and ‘how certain they were about their choice’. The first four series of ‘triangle taste tests’ had only MSG added and the starting concentration was 0·4 % (w/w) MSG. If they could correctly recognise the soup with added 0·4 % MSG for ≥ eight of the triplicates then the concentration was decreased (or if not, it was increased) by a step of 0·1 % until the threshold for the taste of MSG was determined (within the concentration range of 0·1–0·8 %). Thereafter, another series of ‘triangle taste tests’ were administered where the starting concentration of MSG was the lowest concentration previously tasted plus 0·25 % IMP-5. The reason for the second series of triangles was to determine if addition of 0·25 % IMP-5 could lower the concentration of MSG recognised. Between each cup within the ‘triangle taste test’, subjects were encouraged to rinse their mouth with water and chew on plain white bread (but not swallow) to get rid of previous tastes. Moreover, a 10–15 min break was taken between each consecutive set of ‘triangle taste tests’ so that subjects could again rinse their mouth with water or chew on plain white bread to get rid of previous tastes. On each experimental session, subjects required a total of between four and eight ‘triangle taste tests’ to determine their taste threshold; each experimental session took 2–3 h.
Sensory ratings for the taste properties of the soup
Sensory ratings (for example, pleasantness, savoury, meaty, salty, heartiness, sour, sweet, bitter, creamy, fatty) were measured after every ‘triangle taste test’ using a validated 100 mm visual analogue scale(Reference Chapman, Goble, Wittert and Horowitz27). The scale ranged from ‘entirely not’ on the left to ‘extremely’ on the right. For each sensory property, the change in the sensory property score was calculated as the score given during tasting of the soup at the first concentration presented (i.e. 0·4 % MSG) minus their score given at their threshold concentration (their lowest concentration tasted).
‘Liking’ and ‘eating frequency’ scores for protein, fat and carbohydrate food items, and ‘protein preference’ scores
Subjects completed a questionnaire asking them about their ‘liking’ and ‘eating frequency’ of twenty-two high-protein, twenty-two high-fat and twenty-two high-carbohydrate food items (total of sixty-six common food items eaten in the Netherlands)(Reference Kovacs, Lejeune and Westerterp-Plantenga28). For each item on this questionnaire, subjects had to rate ‘How nice do you find…’ using a four-point scale where a score of 1 indicated ‘not at all nice’ and 4 indicated ‘delicious’ and ‘How often do you eat…’ where a score of 1 indicated ‘never’ and 4 indicated ‘at least once per week’. For each macronutrient, the answers were summed to give a ‘liking’ and ‘eating frequency’ score (a score of 88 was the maximum achievable score for any single macronutrient). One's preference for dietary protein was assessed using: (1) ‘the protein liking ratio’ – calculated as the protein liking score divided by the total liking scores of protein+fat+carbohydrate and (2) ‘the eating frequency ratio’ – calculated as the protein eating frequency score divided by the total eating frequency scores of protein+fat+carbohydrate.
Statistical analysis
All data are presented as mean values with their standard errors unless stated otherwise. Statistical analyses were made using SPSS for Windows version 11.5 (SPSS Inc., Chicago, IL, USA), and the criterion for significance (two-tailed) was set at P < 0·05. Outcomes measured on experimental session 1 were compared with those from experimental session 2 using Student's paired t test. Several outcomes (i.e. sensory properties and liking, eating frequency and protein preference scores) were found to be different on day 2 compared with day 1. Accordingly, only data from test day 2 was analysed which should have in turn excluded learning effects. A frequency distribution of thresholds for the taste of MSG ± IMP-5 was determined for the study population. Pearson's correlation and linear regression analyses were performed to examine the relationships between the threshold of MSG ± IMP-5 and (i) the change in sensory properties and (ii) protein liking, eating frequency and preference scores.
Results
Taste threshold of monosodium glutamate alone and in combination with inosine 5′-monophosphate
There was no difference between the lowest concentration of MSG alone recognised on experimental session 1 as compared with experimental session 2 (0·33 (sem 0·04) v. 0·30 (sem 0·03); degrees of freedom = 58; t = 0·81; P = 0·5). There was also no difference between the lowest concentration of MSG+0·25 % IMP-5 identified on experimental session 1 as compared with experimental session 2 (0·30 (sem 0·04) v. 0·23 (sem 0·03); degrees of freedom = 53; t = 1·67; P = 0·1). Fig. 1 shows the frequency distribution of the taste thresholds for all individuals who completed experimental session 2. The percentage frequency of individuals who could taste MSG within the concentration range was 82·8 % when only MSG was added and was 79·3 % when MSG was added in combination with 0·25 % IMP-5. The taste threshold of MSG was lowered from 0·33 (sem 0·24) % to 0·26 (sem 0·22) % when 0·25 % IMP-5 was added (t = 3·25; degrees of freedom = 42; P = 0·002). There was no effect of sex on the lowest concentration of MSG tasted when tested alone (P = 0·7) or in combination with IMP-5 (P>0·38).
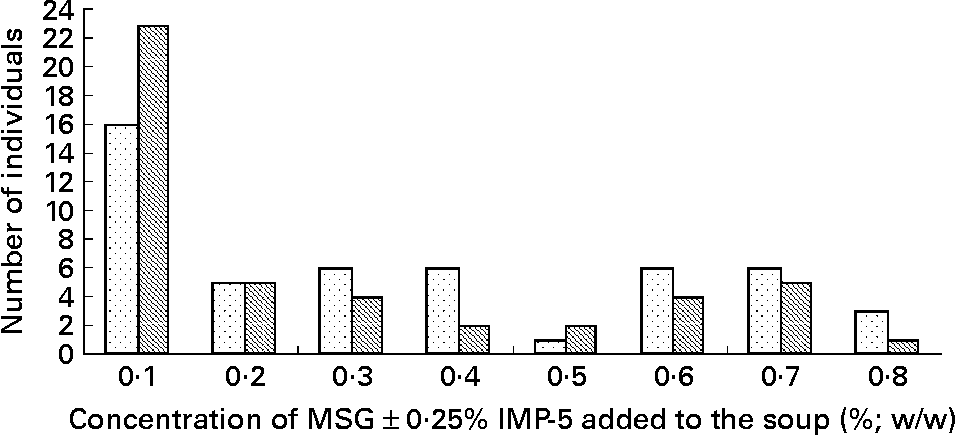
Fig. 1 Frequency distribution of the taste thresholds of monosodium glutamate (MSG) alone (![]() ) and in combination with 0·25 % inosine 5′-monophosphate (IMP-5) (
) and in combination with 0·25 % inosine 5′-monophosphate (IMP-5) (![]() ) for the thirty-five women and twenty-four men who completed experimental session 2. Within the concentration range of 0·1 to 0·8 % (w/w), ten subjects could not taste MSG at all when added alone to soup and twelve subjects could not taste MSG when added in combination with 0·25 % IMP-5; these subjects are not shown in the frequency distribution graph.
) for the thirty-five women and twenty-four men who completed experimental session 2. Within the concentration range of 0·1 to 0·8 % (w/w), ten subjects could not taste MSG at all when added alone to soup and twelve subjects could not taste MSG when added in combination with 0·25 % IMP-5; these subjects are not shown in the frequency distribution graph.
Relationships between the taste threshold of monosodium glutamate and inosine 5′-monophosphate and the sensory properties of the soup
For the overall study population, the taste threshold of MSG+0·25 % IMP-5 was not significantly associated with any of the expected sensory properties (i.e. pleasantness, savoury, meaty, hearty, salty). This finding remained the same even when the non-tasters at any concentration within the 0·1–0·8 % range were excluded from the analysis. A strong association, however, was observed between the taste property ‘meatiness’ and the taste threshold of individuals who correctly identified the presence of MSG in soup when concentrations of ≤ 0·4 % MSG were added (F(1,31) = 5·6; R 0·40; P = 0·02).
The effect of monosodium glutamate and inosine 5′-monophosphate taster threshold on the liking and eating frequency scores of protein, fat and carbohydrate
The ‘liking’ scores were not significantly different for protein, carbohydrate and fat, respectively (59·6 (sem 1·1) v. 64·1 (sem 1·2) v. 61·5 (sem 1·2)) and neither were the ‘eating frequency’ scores (59·6 (sem 1·1) v. 65·3 (sem 1·2) v. 54·8 (sem 1), respectively). ‘Protein preference’ expressed as the ratio of protein ‘liking’ scores over the total scores for all three macronutrients was found to be 0·32 (sem 0·004). When expressed as the ratio of protein ‘eating frequency’ over the total for all three macronutrients, protein preference was 0·33 (sem 0·005).
Relationships between the taste threshold of monosodium glutamate+0·25 % inosine 5′-monophosphate and the ‘liking’, ‘eating frequency’ and ‘preference’ scores of high-protein foods
For the overall study population, the taste threshold of MSG+0·25 % IMP-5 was associated with the ‘liking’ (r − 0·34; P = 0·01) and ‘preference’ (r − 0·31; P < 0·02) scores for high-protein foods (Fig. 2). The ‘eating frequency’ score for protein was not related to the taste threshold for MSG+0·25 % IMP-5. Similar associations were found when the twelve individuals who could not taste any concentration of MSG within the range of 0·1 to 0·8 % were removed (r − 0·34, P = 0·02 for the association between the taste threshold and protein ‘liking’ score and r − 0·31, P = 0·04 for the association between the taste threshold and protein ‘preference’).
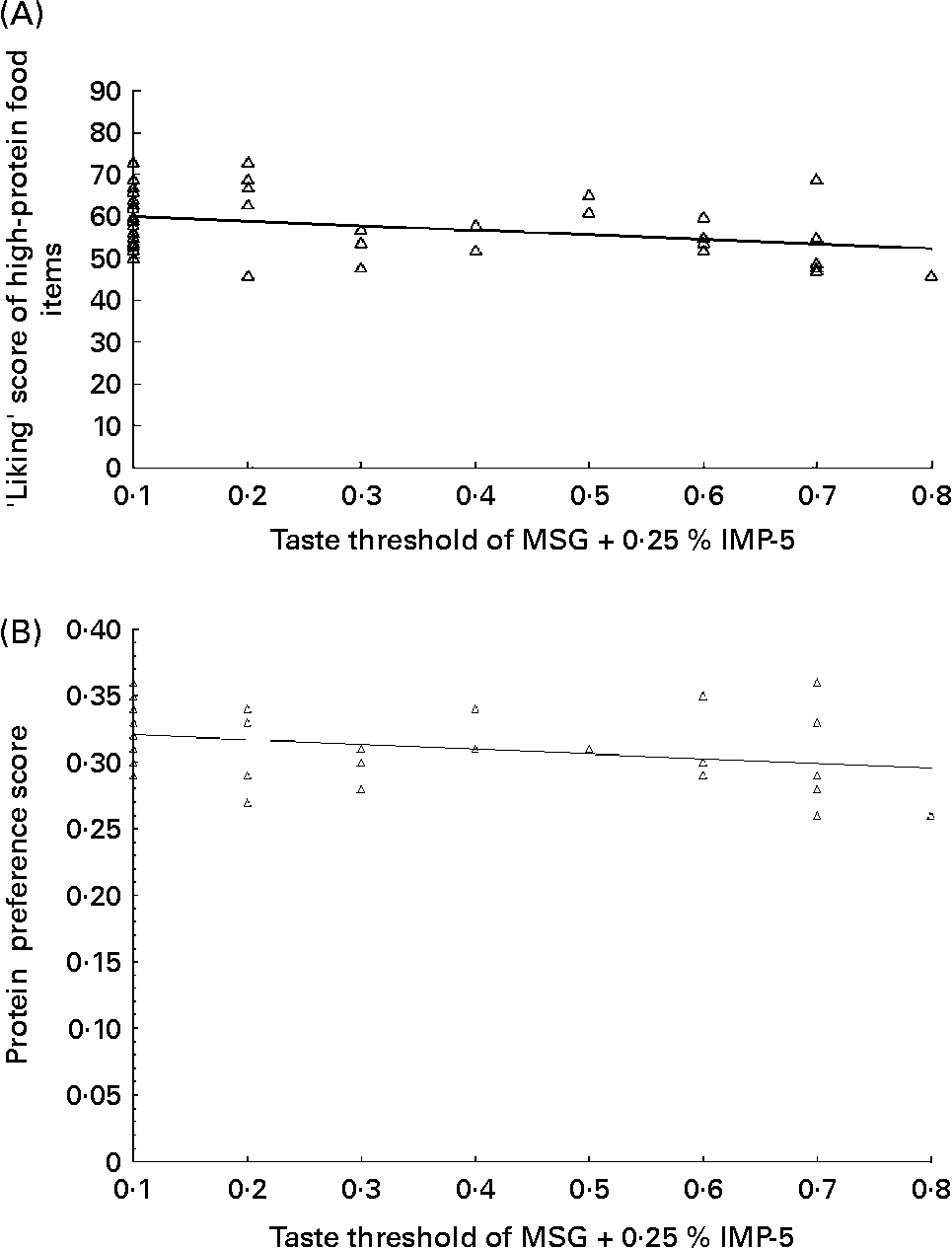
Fig. 2 The taste threshold of monosodium glutamate (MSG), when combined with 0·25 % inosine 5′-monophosphate (IMP-5), was associated with the ‘liking’ score of high-protein foods (F(1,57) = 7·1, R 0·34, P = 0·01) (A) and the ‘protein preference’ score (as assessed using the ratio of ‘liking’ score of protein over the sum of scores for protein, fat and carbohydrate) (F(1,57) = 5·9, R 0·31, P = 0·02)), in the overall study population (B). The relationship of MSG+0·25 % IMP-5 with the ‘liking’ score is y = 60·98 − 10·78 × (concentration of MSG+0·25 % IMP-5); R 2 0·11. The relationship of MSG+0·25 % IMP-5 with the protein preference score is y = 0·32 − 0·03 × (concentration of MSG+0·25 % IMP-5); R 2 0·10.
Discussion
The main findings from the present study are: (i) the taste threshold for MSG alone and in combination with IMP-5 is stable when determined on separate occasions; (ii) addition of IMP to MSG-enriched foods can lower the taste threshold of MSG; (iii) the taste threshold of MSG ± IMP-5 is not associated with sensory properties of taste, the exception being with ‘meatiness’ of taste; (iv) the taste threshold of MSG ± IMP-5 is associated with the ‘liking’ of high-protein foods.
In this Dutch population, the addition of 0·25 % IMP to the MSG-flavoured soup lowered the taste threshold of MSG from 0·33 (sem 0·24) % (w/w) to 0·26 (sem 0·22) %. A previous Dutch study reported that the mean taste threshold of MSG alone was 0·2 % (w/w) for twenty-one elderly men and women (aged 60–75 years) and about 0·15 % (w/w) for twenty-one young men and women (aged 19–33 years)(Reference Mojet, Christ-Hazelhof and Heidema29). They also showed that the threshold of IMP-5 for elderly men was 1 % (w/w) compared with 0·48 % (w/w) for the women and for young men and women it was 0·13 v. 0·24 %. That water was used as the tastant vehicle may explain, at least in part, the different mean taste thresholds of MSG in their study compared with ours. We used a clear vegetable broth as the tastant vehicle because MSG at 0·094–6·0 % concentrations in aqueous solutions have been reported as neutral or unpleasant and are therefore not considered to be representative for testing real-life taste preferences(Reference Bellisle25, Reference Okiyama and Beauchamp30). In a study of young Americans where canned chicken broth was used as the tastant vehicle, the taste threshold of MSG was 0·345 (sem 0·181) %. This was in complete agreement with our findings(Reference Schiffman, Sattely-Miller, Zimmerman, Graham and Erickson31); they did not, however, examine the taste thresholds of MSG in combination with IMP-5. In the present study, the observed improved sensitivity to MSG when IMP-5 was added may have been due, at least in part, to a learning effect given that the MSG+IMP-5 triangle tests always took place after the MSG-alone tests.
For the overall study population, descriptive taste properties such as pleasant, savoury, salty, hearty and meaty were not related to the taste threshold of the MSG+0·25 % IMP-5-enriched soup. This was somewhat surprising given that long-standing research has documented the usefulness of MSG in combination with IMP-5 as a ‘flavour enhancer’(Reference Chaudhari, Landin and Roper4–Reference Prescott10) and reported the use of ‘meatiness’ as a descriptor for the taste of MSG(Reference Okiyama and Beauchamp30). On the other hand, given (i) the existence of multiple glutamate taste receptor types in the taste cells; (ii) the presence of several transduction pathways, and (iii) that Na ions are sensed separately to the glutamate ions, it was not completely unexpected that the overall study population exhibited a large degree of variation in their perceptions of the taste of MSG(Reference Lugaz, Pillias and Faurion32). However, when we narrowed the population to only individuals who correctly identified the presence of MSG when low concentrations were added (0·1 to 0·4 %), we did observe a strong association between ‘meatiness’ and the taste threshold. Since meaty flavours are used to describe different types of animal proteins it can be argued that the taste of MSG may be one compound that represents the taste of dietary protein.
Another interesting finding in the present study was that of an inverse association between the taste threshold and ‘liking’ and ‘preference’ scores for dietary protein for the overall study population. This finding is comparable with that observed for food-adventurous 6-n-popylthiouracil (PROP) tasters who had a greater liking of a variety of strong-tasting bitter, hot and pungent foods than PROP tasters who were less adventurous with their food choices(Reference Ullrich, Touger-Decker, O'Sullivan-Maillet and Tepper33). Such findings lend to cautious speculation by the medical community that dietary therapies recommended to treat certain diseases may be better tailored to the macronutrient preference of the treated individual. We do, however, acknowledge that the perceived pleasure derived from eating foods we like only partially determines our overall food intake and this is definitely reflected by the strength of the association observed in this population; other factors include our immediate psychological and physiological states, and where, when and with whom we are eating(Reference Mela34).
From this study population we conclude that the taste threshold of MSG improves when combined with IMP-5. Moreover, the taste threshold of MSG in combination with IMP-5 was best described by the taste descriptor ‘meatiness’, and it appears to predict the ‘liking’ of and ‘preference’ for high-protein foods, at least in a proportion of subjects within this population. Further investigation in a larger population of individuals is warranted to confirm these relationships.
Acknowledgements
We would like to thank Loek Wouters for analysing the 2H-labelled urine samples for determination of body composition. N. D. L.-M. designed the experiment, collected the data, analysed the data and wrote the manuscript. A. J. P. G. S. read the manuscript and contributed to the discussion. M. S. W.-P. designed the experiment, helped analyse the data and write the manuscript, and supervised the project. None of the authors had any financial or personal interest in any company or organisation sponsoring the research. The study was supported by project funding (RTD line 5, Obesity and Food Technology) from the The European DiOGenes programme 2002–2006 and Top Institute Food and Nutrition, The Netherlands.




