Obesity has reached epidemic proportions in many countries worldwide1. The steady increase in its prevalence has been accompanied, on one hand, by an increase in the consumption of energy-dense food and, on the other, by a reduction in physical activity levels1, Reference Varo, Martinez-Gonzalez, De Irala-Estevez, Kearney, Gibney and Martinez2. The role of exercise in the prevention of positive energy balance and obesity is well recognisedReference King3, Reference Moore4. There is evidence that a sedentary lifestyle predisposes to a failure in appetite regulation, whereby energy intake (EI) is uncoupled from energy expenditure, due to the inability to downregulate dietary intake to match widespread actual levels of inactivityReference Murgatroyd, Goldberg, Leahy, Gilsenan and Prentice5. This suggests a link between inactivity and disrupted homeostatic mechanisms involved in appetite.
Short-term feeding studies using the manipulation of preload energy content to alter energy balance have been used in the area of appetite research to study homeostatic feedback control of hunger/satietyReference Kissileff6, a process that, if not tightly regulated, can lead to energy imbalance. These studies have demonstrated differences in hunger and subsequent food intake following a high-energy preload (HEP) and low-energy preload (LEP)Reference Goldberg, Murgatroyd, McKenns, Heavey and Prentice7–Reference Rolls, Kim-Harris, Fischman, Foltin, Moran and Stoner9. Appetite responses are thus to some extent dependent on previous EI and sensitive to energy deficits induced through differences in dietary intake. In contrast, energy deficits created by exercise induce different effects from those induced by dietReference Hubert, King and Blundell10. Although strong compensatory physiological mechanisms seem to be activated when dietary energy deficits disrupt energy balanceReference Goldberg, Murgatroyd, McKenns, Heavey and Prentice7, Reference Hill, Leathwood and Blundell8, the majority of the studies show little or no effect of an acute bout of exercise on subjective feelings of appetite, satietyReference Hubert, King and Blundell10–Reference Lluch, King and Blundell13 or EIReference Hubert, King and Blundell10–Reference Thompson, Wolfe and Eikelboom18.
However, in contrast to the weak coupling between energy expenditure during acute exercise and subsequent EI in the short term, physically active individuals, such as long-distance runners, usually have a greater EI than those with a sedentary lifestyle but paradoxically have a lower BMI, suggesting that a tight coupling between EI and expenditure exists at high levels of physical activityReference King, Tremblay and Blundell19. This raises the hypothesis that exercise may sensitise the physiological mechanisms involved in appetite control, thereby improving the coupling between EI and expenditure. Support for the role of exercise on appetite control was provided by King et al., who demonstrated a good compensation for differences in EI following periods of exercise in male volunteers who undertook regular exerciseReference King, Appleton, Rogers and Blundell20.
Previous studies in our laboratories, using a cross-sectional design, have shown that active men have a better short-term appetite response to covert preload energy manipulation compared with sedentary menReference Long, Hart and Morgan21. The sedentary group was unable to adjust subsequent EI in response to preload energy manipulation, with buffet EI 60 min after either an HEP or LEP remaining essentially the same. In contrast, the active group decreased their subsequent EI following an HEP, demonstrating an almost perfect (90 %) compensation. Although these findings provide strong but indirect evidence for the beneficial role of exercise in appetite regulation, they do not prove causality, as the observed effects could have been due to lifestyle or other factors in the two groups unrelated to their activity levels.
The primary aim of the present study was therefore to examine whether increasing habitual physical activity levels in sedentary normal-weight volunteers could improve the sensitivity of short-term appetite regulation in response to two covertly manipulated preloads, using a longitudinal design. Secondary aims were to determine whether any improvement in appetite regulation observed with exercise resulted from changes in macronutrient selection and to examine whether the response to exercise differed between genders. Short-term appetite regulation was assessed at baseline and after a 6-week moderate-intensity exercise intervention, using subjective ratings of motivation to eat and objective measures of food intake at a buffet meal and over a 24 h period, following two preloads of different energy content. The present study tested the hypothesis that an increase in habitual physical activity levels in sedentary individuals would result in an improvement in their appetite control, enabling them to detect differences in preload energy manipulation and subsequently to adjust for these.
Materials and methods
Participants
Twenty-nine healthy sedentary volunteers (fifteen men, fourteen women), with a mean age of 29·8 (SD 11·6) years and a mean BMI of 22·7 (SD 2·3) kg/m2, who were not dieting to lose weight, were recruited. A sedentary lifestyle was defined as not being engaged in strenuous work or in regular brisk leisure physical activity more than once a week or engaged in light exercise for more than 20 min/d, more than three times a week. Physical activity and exercise were defined according to the American College of Sports Medicine22. This was assessed through an exercise history of the 3 months prior to the study. Participants' baseline anthropometric, fitness and metabolic data are shown in Table 2 later.
The Dutch Eating Behaviour QuestionnaireReference van Strien, Frijters, Bergers and Defares23 was used to assess restrained, emotional and external eating behaviour. Only those scoring less than 4 on any one of the Dutch Eating Behaviour Questionnaire subscales were accepted for the study. Average scores were of 2·2 (SD 0·7), 2·5 (SD 0·7) and 3·0 (SD 0·5), respectively.
Participants were unaware of the differences in energy between the preloads and of the real purpose of the study; they were told that it aimed to investigate the effects of exercise on mood and food choices. All participants gave written consent before enrolling in the study and were debriefed at the conclusion of the study. The study was approved by the University of Surrey Ethics Committee.
Study protocol
Participants underwent a 6-week moderate-intensity exercise programme. A preload/test-meal paradigm, using an HEP and LEP, was used at baseline and after the exercise intervention to assess macronutrient selection and the ability of participants to regulate their food intake in response to previous dietary intake. The effect of the exercise programme on habitual food intake, anthropometry, cardiovascular fitness and fasting hormones and metabolites was also assessed.
Exercise programme
The exercise programme started at week 1 and ran until week 6. Participants were given two choices: free temporary gym passes, for the duration of the study, or a stationary exercise bicycle delivered to their home. They were asked to perform 30–45 min of moderate aerobic exercise (65–75 % of maximal heart rate) at least four times per week, continuously or in bouts of at least 10 min each, in accordance with the most recent recommendations designed to improve aerobic fitness and body composition24–26.
To ensure proper monitoring of the exercise intensity and duration, and to verify the participants' compliance with the exercise prescription, participants were asked to use a heart rate monitor (Polar F1; Polar Oy, Kempele, Finland) every time they exercised and to record in a daily diary the exercise they performed, including the type, duration and intensity (average heart rate during each bout of exercise as displayed on the heart-rate monitor and self-recorded by each participant). In addition, for those who choose the gym, frequency of attendance was independently obtained from a member of the staff. Moreover, to check that the prescribed exercise did not result in a compensatory reduction in non-exercise physical activity, participants wore pedometers for a week, before the start of the study (pre-study habitual physical activity) and at weeks 3 and 6 of the exercise programme. In order to maintain compliance, all participants were contacted by telephone once a week.
Anthropometric measurements (weight, height and body composition using bioimpedance) were taken at the baseline and end of the exercise programme, together with a fasting venous blood sample for hormone and metabolite analysis. As an independent measure of compliance with the prescribed exercise programme, maximal oxygen uptake was estimated using a submaximal exercise test on a cycle ergometer (YMCA protocol)22, at the beginning and end of the exercise intervention.
Preloads and measurement of appetite
Two different preloads, HEP and LEP, with similar sensory properties were used in this study. They were presented as 450 ml flavoured milkshakes differing in their energy content by 361 kcal through the addition of maltodextrin (Table 1).
Table 1 Ingredients and nutritional composition of the preloads
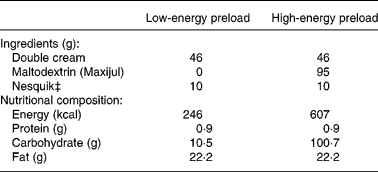
Amounts and nutritional composition per 450 ml serving, made up to this volume with water.
‡ Chocolate, strawberry or banana flavoured.
Food intake after preload consumption was assessed using a standardised buffet ad libitum, in excess of expected consumption (4100 kcal, 85 g protein, 250 g fat, 373 g carbohydrates), with a variety of lunch-type foods (sandwiches, salad, fruit, cake, biscuits, crisps, yogurt, mayonnaise, mustard). Participants were asked to rank different food items in each one of the above categories by order of preference. They were then offered their second food option within each category and second and third option of sandwich fillingsReference Rogers, Hindmarch and Stonier27. Foods were purchased from a local supermarket and were not modified in any way. Small bite portions of fixed size (one quarter of a sandwich, sliced banana, etc.) were used to deprive participants of familiar cognitive and visual clues used to self-monitor food consumptionReference Stubbs, Johnstone, O'Reilly and Poppitt28.
Food was weighed and/or counted before participants sat down to the meal and reweighed/re-counted after each subject had finished eating, to allow calculations of EI and macronutrient intake. All dietary analysis was performed using Windows Diets Research Version programme (Robert Gordon University, Aberdeen, UK). Participants were presented with exactly the same type and amount of food on all of the four appetite-challenge days.
Subjective feelings of hunger (‘How hungry do you feel?’) and fullness (‘How full do you feel?’), as well as the palatability of the preloads, were assessed using 10 cm self-rated visual analogue scales (VAS) as previously describedReference Hill, Leathwood and Blundell8.
Appetite-challenge days
Using a randomised single-blind cross-over design, participants were given an HEP or LEP at baseline on different days of the week (appetite-challenge days), at least 2 d apart in order to avoid participants from becoming bored with the foods presented at the buffet and to prevent any cross-over effects. This was again repeated after the 6-week exercise intervention, with participants acting as their own controls.
On the morning of each appetite-challenge day, participants were asked to consume their usual breakfast before 09.30 hours. This was the same on all four occasions in order to standardise pre-study appetite. After that, participants were instructed not to eat or drink anything except water, which was permitted up to 10.30 hours, and were asked to arrive at the Clinical Investigation Unit at 11.45 hours. Participants were also asked to avoid alcohol consumption and exercise during the 24 h prior to, and during, each appetite-challenge day.
Participants sat quietly after arrival, and instructions regarding the completion of the VAS were provided. The first VAS was completed to assess baseline hunger/fullness feelings. Preloads were then presented, and participants were asked to consume them within 5 min. Further VAS were completed immediately after the preload (including the question ‘How would you rate the palatability of the milkshake?’) and then at 20, 40 and 60 min. During this period of time, participants stayed in the Clinical Investigation Unit, but they were free to write, read or watch television. Participants were free to talk to each other, but were asked not to discuss the study or their VAS scores. Each VAS was collected before the next was given to avoid participants modifying their previous scores.
A buffet meal was served 60 min after the preload, and participants, each confined to an individual booth, were instructed to eat until comfortably full. To avoid opportunistic overconsumption, participants were informed that they could take home any food left over after the buffet lunch. Participants were also asked to record in a food diary all they consumed after the buffet lunch until (and including) breakfast on the following day (post-buffet food diary) in order to estimate 24 h energy compensation following the preloads.
Food intake
Participants were asked to maintain their normal diet throughout the study. This was verified by a 3 d estimated food diary (including at least one weekend day), using household measures, at baseline and week 6 of the exercise intervention. A 24 h food recall was performed prior to each appetite-challenge day in order to verify participants' compliance with the study instructions.
Biochemical analysis
Fasting blood samples were analysed for total and HDL-cholesterol, triacylglycerol, NEFA, glucose and insulin. Glucose was analysed using an immobilised enzyme biosensor (YSI 2300 Stat Plus Glucose and Lactate Analyzer; Yellowsprings, OH, USA), insulin using an ELISA (MTL Research Limited, Cardiff, UK), and all other biomarkers were quantified using a Randox Space automated system (AlfaWasserMann; Randox, Co. Antrim, UK). The interassay CV were less than 5 % for metabolites and less than 10 % for insulin. Insulin sensitivity was calculated using the homeostatic model assessment (HOMA model)Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner29.
Statistical analysis
Statistical analysis was carried out using SPSS (SPSS Inc., Chicago, IL, USA). All variables were checked regarding their normal distribution using the Shapiro–Wilk test. Statistical significance was assumed at P < 0·05, unless otherwise stated.
Differences in energy and macronutrient intake in the 24 h before each appetite-challenge day (from data derived from the food histories) were assessed by repeated-measures ANOVA.
Changes in habitual energy and macronutrient intake with the exercise programme (based on food diaries), as well as in anthropometric variables, fitness and circulating metabolites, were assessed by paired sample t tests.
The effect of preload (HEP v. LEP), exercise (pre v. post) and gender on energy and macronutrient (in percentage) intake, at the buffet lunch and over a 24 h period (cumulative EI), was assessed by a mixed between-within participants ANOVA with preload and exercise as the within-subject variables and gender as the between-subjects factor. Additionally, paired sample t tests were performed to investigate differences in buffet EI after each preload before and after the 6-week exercise intervention. In order to correct for multiple comparisons, the level of significance (α level) was reduced to P < 0·01. Energy compensation, also known as the compensation index, was calculated as the difference in EI between the two study days (HEP v. LEP) divided by the difference in preload energy content and expressed as a percentageReference Johnson and Birch30. This was calculated for both EI at the buffet lunch and cumulative EI over a 24 h period following preload consumption.
The effect of time, preload (HEP v. LEP) and gender on subjective feelings of hunger/fullness was assessed separately at baseline and the end of the study by a mixed between-within subjects ANOVA, with preload and time as the within-subject variables and gender as the between-subjects factor.
Results
Anthropometry, fitness and metabolic profile
Of the twenty-nine participants initially recruited, four men did not complete the study due to individual work pressures. The results are, therefore, presented for twenty-five participants (eleven men, fourteen women). BMI, percentage of body fat and fitness levels were not significantly different between the four men who withdrew and the eleven who completed the exercise intervention. Changes in anthropometry, fitness and metabolic profile are shown in Table 2. No significant changes were observed in any of the anthropometric measures or blood metabolites analysed with the exercise intervention. The majority of the participants had a fitness level, based on their estimated maximal oxygen uptake, below the thirtieth percentile at baseline according to the American College of Sports Medicine22, and significant improvements in fitness were observed with the exercise intervention in all participants (P < 0·001), men (P < 0·01) and women (P < 0·05).
Table 2 Anthropometry, fitness levels and metabolic profile at baseline and at the end of the study in all participants (n 25), men (n 11) and women (n 14). Results expressed as mean ± sd

HOMA, homeostatic model assessment, calculated as: (fasting insulin levels (μU/l) × fasting glucose levels (mmol/l)) / 22·529.
‡ Percentile of maximal oxygen uptake (ml/kg/min) adjusted for age and gender: classification according to the American College of Sports and Medicine, 2000.
Mean values were significantly differenct between baseline and the end of the study: *P < 0·05, **P < 0·01, †P < 0·0001.
As expected, men were significantly heavier and had a lower percentage of body fat than women at both the baseline and end of the study; they had a significantly higher maximal oxygen uptake (ml/Kg per min) at the end and a bigger improvement in fitness levels (5·2 (SD 4·7) v 1·6 (SD 2·3); P < 0·05).
Exercise compliance
Compliance with the prescribed exercise programme was very good. The whole group exercised on average 4·0 ± 0·8 times per week, with a total of 187·3 (SD 74·9) min/week and at an intensity of 69·9 (SD 3·1) % of their maximal heart rate. The only significant differences observed between genders was a higher frequency of exercise in women compared with men (4·4 (SD 0·8) v. 3·6 (SD 0·6) times a week; P < 0·05).
The number of steps per week, for the whole group, was 8474 (SD 2819) before the start of the study, 7996 (SD 2929) at week 3 and 7806 (SD 2956) at week 6, with no significant differences over time or between genders.
Food histories and food diaries
The exercise intervention did not cause any significant change in daily energy or macronutrient intake (as assessed by the 3 d food diaries), when comparing preintervention with week 6 of the exercise programme in either the whole group, men or women. In addition, energy and macronutrient intake for the 24 h preceeding each of the four appetite-challenge days was not significantly different, in either the whole group, men or women.
Energy and macronutrient intake over 24 h
Cumulative EI for the 24 h following preload consumption, before and after the 6-week exercise intervention, is shown in Fig. 1. ANOVA showed a significant activity × preload interaction (P = 0·023) on 24 h cumulative EI, but no significant effect of exercise, preload or exercise × preload × gender interaction.

Fig. 1 Cumulative EI after the high-energy (HEP; □) and low-energy (LEP; ![]() ) preload, at baseline and at the end of the study. Mean values with their standard errors for twenty-five participants (eleven men and fourteen women). ANOVA showed significance for exercise × preload interaction (P = 0·023), but no significant main effects of exercise or preload or an exercise × preload × gender interaction. Mean values were significantly different between conditions: **P < 0·01.
) preload, at baseline and at the end of the study. Mean values with their standard errors for twenty-five participants (eleven men and fourteen women). ANOVA showed significance for exercise × preload interaction (P = 0·023), but no significant main effects of exercise or preload or an exercise × preload × gender interaction. Mean values were significantly different between conditions: **P < 0·01.
Paired t tests comparing cumulative EI following preload consumption (assuming a α level of less than 0·01 to correct for multiple comparisons) found no significant differences in cumulative EI (kcal) after the HEP and LEP at baseline (2172 (SD 785) v. 2163 (SD 627); P = 0·925). After the 6-week exercise intervention, however, buffet EI after the HEP was significantly lower than after the LEP (18 301 (SD 638) v. 2162 (SD 594); P = 0·007) for the whole sample. A similar baseline pattern was observed when men and women were analysed separately, with no significant differences in cumulative EI after each preload (HEP v. LEP) at baseline (2770 (SD 666) v. 2665 (SD 431) kcal, P = 0·584; 1745 (SD 123) v. 1805 (SD 485) kcal, P = 0·475; for men and women, respectively). After the 6-week exercise intervention, there was a trend towards a lower cumulative EI after the HEP than after the LEP (2013 (SD 733) v. 2444 (SD 657) kcal, P = 0·061; 1677 (SD 526) v. 1924 (SD 428), P = 0·051; for men and women, respectively).
When energy compensation was calculated as described in the Methods section earlier (compensation index, expressed as a percentage), over a 24 h period, a trend to improved compensation was observed with the exercise intervention (8·9 ± 118·5 % at baseline v. 79·5 (SD 146·4) 4 % following the exercise intervention; P = 0·056). When each gender was analysed separately, however, no significant effects were found (for men, 8·4 (SD 157·7) % v. 95·6 (SD 190·7) %, P = 0·150; for women, 20·9 (SD 87·3) % v. 68·4 (SD 113·8) %, P = 0·250; at baseline and end, respectively).
No significant effects of exercise, preload, gender or interactions were observed in the percentage of 24 h cumulative energy provided by each macronutrient.
Energy and macronutrient intake at the buffet lunch
Buffet lunch EI following each preload, before and after the exercise intervention, is shown in Fig. 2. ANOVA showed a significant effect of preload on buffet EI (kcal), with buffet EI after the LEP being significantly higher than that after the HEP (858·4 (SD 284·2) v. 695·1 (SD 294·3) kcal; P < 0·0001). No significant effect of exercise, preload × exercise interaction or preload × exercise × gender interaction was observed on buffet EI.
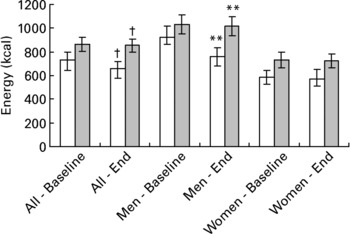
Fig. 2 Energy intake (kcal) at the buffet lunch after the high-energy (HEP; □) and low-energy (LEP; ![]() ) preload, at baseline and end of the study. Mean values with their standard errors for twenty-five participants (eleven men and fourteen women). ANOVA showed a significant effect of preload (P < 0·0001) but no significant effect of exercise, preload × exercise or preload × exercise × gender interaction. Mean values were significantly different between conditions: **P < 0·01, †P < 0·0001 (assuming α < 0·01 to correct for multiple comparisons).
) preload, at baseline and end of the study. Mean values with their standard errors for twenty-five participants (eleven men and fourteen women). ANOVA showed a significant effect of preload (P < 0·0001) but no significant effect of exercise, preload × exercise or preload × exercise × gender interaction. Mean values were significantly different between conditions: **P < 0·01, †P < 0·0001 (assuming α < 0·01 to correct for multiple comparisons).
Paired t tests comparing buffet EI following preload consumption (assuming an α level of less than 0·01 to correct for multiple comparisons) found no significant differences in buffet EI (kcal) after the LEP and HEP at baseline in all participants (864 (SD 294) v. 735 (SD 302); P = 0·015), men (1031 (SD 269) v. 920 (SD 307); P = 0·28) and women (733 (SD 248) v. 589 (SD 208); P = 0·012). After the 6-week exercise intervention however, EI after the HEP was significantly lower than that after the LEP in all participants (656 (SD 288) v. 853 (SD 280); P < 0·0001) and men (1016 (SD 275) v. 759 (SD 275); P = 0·001), but not in women (724·2 (SD 216) v. 573 (SD 293); P = 0·038).
No significant improvement in energy compensation (compensation index), at the buffet meal, was observed with the exercise intervention in either all participants (35·8 (SD 68·7) % v. 54·6 (SD 60·7) %; P = 0·264), men (31 (SD 89) % v. 71 (SD 49) %; P = 0·225) or women (40 (SD 51) % v. 42 (SD 68) %; P = 0·908).
No significant effects of exercise, preload, gender or interactions were observed in the percentage of energy provided by each macronutrient at the buffet lunch, with the exception of protein, which had a significantly bigger contribution (as a percentage) to buffet EI after the exercise intervention compared with baseline (13·1 (sd 2·6) % v. post 13·9 (sd 3·2) %; P = 0·025 for all participants).
When participants who improved in short-term energy compensation with the 6-week exercise intervention (seven men and eight women) were compared with those who did not improve (four men and six women) in relation to anthropometry, fitness levels, number of steps per day, eating behaviour, exercise compliance and metabolic profile, only the number of steps per day before the start of the study and the duration of exercise (min/week) in men were significantly different. Men who improved in their ability to compensate reported a significantly higher number of steps per day before the start of the study (9917 (SD 3594) v. 5681 (SD 1356); P < 0·05) and a higher duration of exercise (min/week; 173 (SD 18) v. 130 (SD 25) min/week; P < 0·05), during the 6-week exercise programme, compared with those who did not improve.
Changes in subjective ratings of hunger/fullness and palatability of the preload
Subjective hunger and fullness immediately before and for 55 min after preload consumption can be seen in Tables 3 and 4. A significant effect of time (P < 0·0001), but no significant effect of preload, gender or interactions, was found in hunger scores, which decreased immediately following preload intake and thereafter increased progressively over time both at baseline and at the end of the study.
Table 3 Subjective ratings of hunger (cm) at intervals before and after high- (HEP) and low-energy (LEP) preload consumption, at baseline and at the end of the study, in all participants (n 25), men (n 11) and women (n 14)

ANOVA showed a significant effect of time (P < 0·0001) both at baseline and end of the study, but no effect of preload, gender or interactions.
Table 4 Subjective ratings of fullness (cm) at intervals before and after high- (HEP) and low-energy (LEP) preload consumption, at baseline and at the end of the study, in all participants (n 25), men (n 11) and women (n 14)

ANOVA showed a significant effect of time (P < 0·0001), both at baseline and the end of the study, and a significant effect of preload at baseline alone.
Fullness ratings presented an inverse temporal pattern, increasing immediately following preload intake and thereafter decreasing progressively over time (P < 0·0001), both at baseline and at the end of the study. A significant effect of preload was also found at beseline alone, with high higher scores after the HEP compared with the LEP.
No significant differences were observed in palatability ratings between the two preloads, either at baseline or end of the study in either men or women.
Discussion
The main purpose of this study was to examine, using a longitudinal design, the effect of a 6-week exercise programme on short-term appetite control in sedentary individuals. The present study supports the results of our previous cross-sectional studyReference Long, Hart and Morgan21, showing for the first time that exercise improves short-term appetite regulation by leading to a more sensitive eating behaviour in response to previous EI.
A significant improvement in compensation over a 24 h period was observed with the exercise intervention, as evidenced by the significant exercise × preload interaction. Although at baseline participants did not distinguish between the two preloads and had a similar cumulative EI over the next 24 h, after the 6-week exercise intervention they significantly downregulated cumulative EI after the HEP compared with the LEP (see Fig. 1 earlier). All macronutrients seem to have contributed to this improvement in compensation over a 24 h period. Moreover, a near-significant (P = 0·056) improvement in energy compensation (expressed as a percentage compensation over the 24 h period), from an average of 8·7 % to 79·5 %, was observed with exercise intervention.
This improvement in compensation on the first 24 h following preload intake was not reflected acutely at the buffet meal. ANOVA did not reveal an exercise × preload interaction on buffet EI. Secondary analysis, however, showed that although buffet EI after each preload was very similar at baseline, whether the analysis was performed in all participants, men or women separately, after the exercise intervention buffet EI after the HEP was significantly lower than that after the LEP in all participants and men, but not in women. A gender difference in the appetite response to exercise has been reported by othersReference Imbeault, Saint-Pierre, Almeras and Tremblay11, Reference Thompson, Wolfe and Eikelboom18, Reference Pomerleau, Imbeault, Parker and Doucet31. Although our EI findings are consistent with a gender difference, with men showing a better acute compensatory response to preload manipulation, an exercise × preload × gender interaction was not found, excluding a significant effect of gender on energy compensation at the buffet lunch.
The trend towards an improvement in acute appetite regulation in response to preloading was not, however, paralleled by any significant improvement in the compensation index (energy compensation expressed as a percentage). Our study was not, however, powered to look at changes in the compensation index. The very large interindividual variation in energy compensation (observed in our study, both at baseline and in response to exercise) has been highlighted by othersReference Johnson and Birch30, Reference Cecil, Palmer and Wrieden32. Cecil et al. Reference Cecil, Palmer and Wrieden32 demonstrated that, in children, intraindividual variation in the compensation index was lower relative to their interindividual variation. It is not known whether the same holds true for adults, and what impact exercise might have on intraindividual variation in the compensation index. Our findings support those of previous workers and clearly demonstrate the complexity of the compensation mechanism and the involvement of factors other than exercise in appetite regulation.
We found an average baseline acute energy compensation, at the buffet lunch, of 31 % in our male group. This value is much higher than the 7 % reported in the inactive group in our previous studyReference Long, Hart and Morgan21. Several factors may help to explain these differences, namely eating behaviour and body weight, previously shown to be key determinants in the compensation for previous EIReference Rolls, Kim-Harris, Fischman, Foltin, Moran and Stoner9, Reference Almeras, Lavallee, Despres, Bouchard and Tremblay33–Reference Ruderman35. Although restrained eating, as assessed by the Dutch Eating Behaviour Questionnaire, and BMI were very similar in our own and Long's male inactive group (2·0 (SD 0·8) v. 1·9 (SD 0·5) and 23·4 (SD 2·4) v. 24·3 (SD 3·0) kg/m2, respectively), the slightly lower BMI in our males may help to explain their higher baseline compensation.
After the 6-week exercise programme, our male group showed a 71 % acute energy compensation, a figure that is lower than the 90 % compensation reported in Long's active groups. It is important to emphasise that, in Long's study, participants were classified as active or inactive based on an exercise history of the previous 3 months. We used a similar approach to recruit our sedentary participants. Our exercise programme was, however, of only 6 weeks, which means that, at the end of the study, our participants would still be classified as inactive according to Long's criteriaReference Long, Hart and Morgan21. Therefore, a longer intervention would probably be needed in order to attain that level of energy compensation at the buffet meal. This is supported by the finding that those men who improved in energy compensation acutely at the buffet meal were the more active ones before the start of the intervention (based on pedometer readings) and those who exercised for longer periods during the 6-week exercise programme. These findings strengthen our hypothesis for a role of physical activity on short-term appetite control.
The effects of exercise on appetite control could possibly be explained by its impact on macronutrient preferences and food choices, in the hedonic (pleasure) response to food or, alternatively, in the sensitivity of the satiety cascade systemReference Lluch, King and Blundell13, Reference Blundell, Stubbs, Hughes, Whybrow and King36. We did not, however, find any change in macronutrient selection with exercise (either at the buffet meal or from the 3 d food diaries), which is consistent with the majority of the literatureReference Tremblay and Drapeau37–Reference Donnelly, Kirk, Jacobsen, Hill, Sullivan and Johnson39, and the hedonic response to food was beyond the scope of this study. The mechanisms targeted by exercise that may help to explain its role in appetite regulation are likely to fall into three categories: long-term, including leptin and insulin; intermediate, including post-absorptive signals associated with macronutrient oxidation such as glucose and free fatty acids levels; and, finally, short-term mechanisms involving post-ingestive, but preabsorptive, signals arising from the gastrointestinal tract in response to food intakeReference Blundell40.
Gastrointestinal hormones involved in short-term appetite regulation, such as cholecystokinin, glucose like-peptide-1 and peptide YY, are potential candidatesReference de Graaf, Blom, Smeets, Stafleu and Hendriks41. We did not find any significant changes in fasting insulin, glucose or NEFA levels, or insulin sensitivity (homeostatic model assessment model) with the exercise intervention, and leptin, although not measured, is not expected to have changed as no significant changes in body weight or body composition were observed in our participants. This leaves us with the short-term preabsorptive satiety signs released by the gastrointestinal tract.
To our knowledge, the effect of long-term exercise on the plasma levels of gastrointestinal hormones involved in appetite control, as well as on neuropeptide Y and α-melanocyte-stimulating hormone, the two main neurotransmitters within the hypothalamus involved in the regulation of feeding behaviourReference Murphy and Bloom42, Reference Batterham and Bloom43, remain unknown, with the exception of a single report showing no effect of chronic exercise in fasting cholecystokinin levels in active menReference Bailey, Davies, Castell, Newsholme and Calam44. Although some of these peptides may remain elevated for 3–5 h after a meal, such as cholecystokinin, most start to fall after the first 2–3 hReference de Graaf, Blom, Smeets, Stafleu and Hendriks41. Changes in the plasma levels of these gut peptides could therefore explain the trend towards the improvement in acute compensation at the buffet meal, presented 60 min after preload consumption but are unlikely to account for the significant improvement in energy compensation over 24 h.
The improvement in short-term appetite regulation with the exercise intervention does not seem to have been driven by an improvement in the sensitivity to hunger or satiety signals; although fullness scores were significantly different between preloads before the exercise intervention, that difference disappeared or became smaller afterwards.
Although compliance with the exercise intervention was very good, measurement of compliance is probably the major limitation of this study, since it is based on self-reporting. However, the magnitude of the improvement in estimated maximal oxygen uptake observed with our 6-week exercise programme (10 %) is in line with previous studies in which similar exercise interventions were usedReference Nishida, Higaki and Tokuyama45–Reference Asikainen, Miilunpalo and Oja47. We are aware that the increase in total energy expenditure (EE) induced by exercise presupposes that normal activity throughout the rest of the day remains unchanged or increasesReference Wilmore48, a question that remains controversialReference Meijer, Jansen, Westerterp, Verhoeven, Saris and ten Hoor49, Reference Goran and Poehlman50. We were able to show that normal activity throughout the day, as assessed by pedometers, remained unchanged over time, strongly suggesting an increase in total EE with the exercise programme.
The 6-week exercise programme was not followed by changes in either body weight or body composition. Our failure to find any compensatory increase in habitual EI was, therefore, unexpected, given the increase in total EE. This highlights the limitations of estimating food intake from questionnaires and suggests a degree of underreporting in this sample.
To the best of our knowledge, this is the first study showing that increasing habitual physical activity levels in previously sedentary individuals has an impact on short-term appetite regulation, and that physical activity may not only increase EE, but also lead to a more sensitive eating behaviour in response to previous EI. These findings provide the foundation to future work in this area; they also have important implications in terms of the steadily increasing prevalence of obesity in the UK and the current failure to meet the Department of Health physical activity recommendations26, which were very similar to those used in this research. Further studies are needed to elucidate the optimal intensity and duration of exercise necessary and the mechanisms whereby exercise improves short-term appetite control.
Acknowledgements
Catia Martins was supported by a PhD grant (SFRD/BD/16 294/2004) from Fundação para a Ciência e Tecnologia (Portugal) under the 3rd European Union community support programme








