Higher levels of fruit and vegetable intake have been associated with a decreased risk of various chronic medical conditions(Reference Fairfield and Fletcher1). The antioxidant properties of carotenoids, commonly found in fruits and vegetables, are one explanation for this finding. Increased dietary carotenoid consumption and levels of individual plasma carotenoids may be associated with the decreased risk of cancer and CVD(Reference Mares-Perlman, Millen, Ficek and Hankinson2, Reference Voutilainen, Nurmi, Mursu and Rissanen3). In addition, levels of individual plasma carotenoids have been associated with specific risk factors for chronic medical conditions such as diet, exercise and cholesterol(Reference Brady, Mares-Perlman, Bowen and Stacewicz-Sapuntzakis4–Reference Rock, Thornquist, Neuhouser, Kristal, Neumark-Sztainer, Cooper, Patterson and Cheskin7). Some authors have suggested using total plasma carotenoids as a marker of a diet high in fruits and vegetables(Reference Le Marchand, Hankin, Carter, Essling, Luffey, Franke, Wilkens, Cooney and Kolonel8). However, there are comparatively fewer data regarding total plasma carotenoids and the frequency of both traditional and more novel risk factors for chronic medical conditions. Therefore, we examined the association between various traditional and more novel risk factors with total plasma carotenoids in a cross-sectional sample of middle-aged and older men.
Methods
Study population
The Physicians' Health Study (PHS) has previously been described in detail(Reference Hennekens, Buring and Manson9). Briefly, the PHS began in 1982 as a randomised, double-blind, placebo-controlled, 2 × 2 factorial trial of β-carotene and aspirin in 22 071 initially healthy male US physicians in the primary prevention of cancer and CVD. All protocols were approved by the Institutional Review Board of Brigham and Women's Hospital.
Blood collection
A follow-up blood collection was conducted among 18 764 randomised men still alive starting in December 1995. Blood kits with vacuum tubes containing EDTA, instructions for the blood draws, and coldpacks were mailed to all 18 764 participants. Physicians returned blood samples in provided coldpacks by prepaid overnight courier. Upon receipt, each sample was centrifuged, divided into samples, and stored at − 82°C. Follow-up blood kits were received from 11 718 (62 %) of 18 764 participants alive at the time of the second blood collection beginning in 1995. A total of 492 middle-aged and older men who contributed blood and were identified as free of CVD and cancer were selected for a nested case–control study of plasma carotenoids and CVD(Reference Sesso, Buring, Norkus and Gaziano10). We performed a cross-sectional analysis on these men identified as controls for this previous study.
Blood samples were analysed for lycopene, β-cryptoxanthin, α-carotene, β-carotene, lutein, zeaxanthin, α-tocopherol and γ-tocopherol at Our Lady of Mercy Medical Center (Bronx, NY, USA), a laboratory that has participated in the US Quality Assurance Program. All assays were quantified by reversed-phase HPLC after extraction and concentration by conventional methods(Reference Sowell, Huff, Yeager, Caudill and Gunter11). Mean intra-assay CV based on blinded quality-control samples ranged from 7·6 % for α-carotene to 11·9 % for α-tocopherol. Plasma lipids, including total cholesterol, HDL-cholesterol (HDL-C) and LDL-cholesterol (LDL-C) were also assayed using commercially available diagnostic kits (Sigma-Aldrich Chemical Co., St Louis, MO, USA) and conventional methods(Reference Allain, Poon, Chan, Richmond and Fu12, Reference Roeschlau, Berut and Gruber13). Levels of C-reactive protein (CRP) were assayed using a validated, high-sensitivity assay (Denka Seiken Company, Tokyo, Japan). Levels of IL-6 and intercellular adhesion molecule-1 (ICAM-1) were assayed using commercially available enzyme-linked immunoadsorbent assays (R&D Systems; Minneapolis, MN, USA).
Baseline characteristics
In addition to returning a blood sample, participants completed a comprehensive questionnaire that included information on self-reported clinical and lifestyle characteristics. For those who did not return a comprehensive questionnaire at the same time as their blood sample, we used questionnaires that were completed closest to the time of blood collection, within a few years, to ensure the complete assessment of potential confounding for our analyses. Questionnaires provided information on age (years), weight and height (converted to BMI; kg/m2), smoking status (categorised as current, former, or never), alcohol use (categorised as rarely or never, monthly, weekly, or daily), frequency of exercise (categorised as rarely or never, < 3 d per week, 3–4 d per week, or ≥ 5 d per week), history of hypertension (yes or no), history of hyperlipidaemia (yes or no), history of diabetes mellitus (yes or no) and systolic blood pressure (mmHg).
Data analysis
Total plasma carotenoids were defined as the sum of plasma α-carotene, β-carotene, lycopene, zeaxanthin, lutein and β-cryptoxanthin. We defined quartiles of total plasma carotenoids based upon their distribution among controls: < 0·995, 0·995–1·300, 1·301–1·715 and >1·715 μmol/l. We first compared participants for clinical and lifestyle characteristics by quartile of total plasma carotenoids. Crude Spearman correlation tests were first calculated for total and individual carotenoids, followed by adjustment for age and total cholesterol. Linear regression models then calculated the parameter estimate and 95 % CI for levels of total plasma carotenoids and each variable after adjustment for total cholesterol. Subsequent models further adjusted for three classes of variables – clinical, lifestyle and biomarkers – as well as a model that included all covariates. We also considered forward, backward and stepwise selection procedures to identify which combination of variables provided the best and most efficient fit of the data.
Next, we used logistic regression models to calculate the OR and 95 % CI for having total plasma carotenoids that are above v. below the median value (1·301 μmol/l). We followed a similar model-building strategy as described above. We considered full multivariate models comparing the upper quartile of total plasma carotenoids (>1·715 μmol/l) with the bottom quartile of total plasma carotenoids ( < 0·950 μmol/l). Finally, we considered the use of a multivitamin in our multivariate models. All statistical analyses were performed using SAS (version 9.1; Cary, NC, USA).
Results
For all 492 men, the mean age in this analysis was 69·6 (sd 8·0) years. For individual carotenoids, the mean levels of each individual carotenoid were as follows: lycopene, 0·18 (sd 0·09) μmol/l; β-cryptoxanthin, 0·20 (sd 0·15) μmol/l; lutein, 0·28 (sd 0·12) μmol/l; zeaxanthin, 0·09 (sd 0·05) μmol/l; α-carotene, 0·14 (sd 0·13) μmol/l; β-carotene, 0·56 (sd 0·51) μmol/l. The mean level of total plasma carotenoids for the study population was 1·45 (sd 0·71) μmol/l. Table 1 presents the characteristics of the population by quartiles of total plasma carotenoids. Increasing levels of total plasma carotenoids were not associated with age or BMI. Significantly fewer men had a diagnosis of hypertension, smoked or drank alcohol with increasing levels of total plasma carotenoids. Total cholesterol, LDL-C and HDL-C each significantly increased with higher quartiles of total plasma carotenoids. Finally, as would be expected, individual plasma carotenoids significantly increased with increasing levels of total plasma carotenoids.
Table 1 Characteristics of 492 middle-aged and older male physicians by quartiles of total plasma carotenoids*
(Mean values and standard deviations for 123 subjects per quartile)

LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1.
* For details of procedures, see Methods.
Table 2 presents Spearman correlations among individual and total plasma carotenoids after controlling for total cholesterol and age. No significant differences were found for crude v. adjusted Spearman correlation coefficients. Individual plasma carotenoids were significantly correlated with one another as well as total plasma carotenoids. After zeaxanthin and lutein, the next highest Spearman correlation among individual carotenoids occurred between α-carotene and β-carotene (r 0·66; P < 0·01).
Table 2 Spearman correlations for plasma levels of each individual carotenoid and total carotenoids controlled for total cholesterol and age among middle-aged and older male physicians*
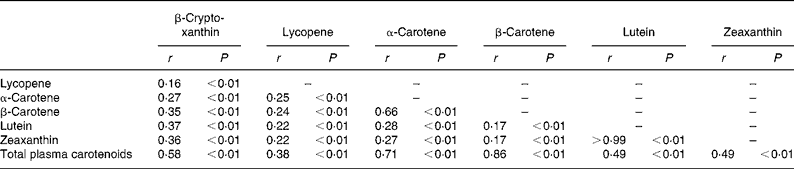
* For details of procedures and subjects, see Methods and Table 1.
Forward, backward and stepwise selection methods were used to generate and compare linear regression models that may predict total plasma carotenoids. The maximum r 2 in each selection process was 0·17 with total cholesterol, LDL-C, HDL-C, BMI, daily alcohol ingestion, weekly alcohol ingestion, current smoking, diagnosis of hypertension, ICAM-1, α-tocopherol and γ-tocopherol statistically significant in each model for total plasma carotenoids (data not shown). Table 3 presents results from the linear regression models for total plasma carotenoids. Among the clinical variables, diagnoses of diabetes mellitus and hyperlipidaemia were not significant predictors of increased total plasma carotenoids in any model, whereas a diagnosis of hypertension and BMI were significant predictors in all three models. Among the lifestyle variables, daily and weekly alcohol intakes were significant predictors of increased total plasma carotenoids in all three models. Among the biomarkers, ICAM-1, HDL-C and α-tocopherol were significant predictors of increased total plasma carotenoids in all three models.
Table 3 Parameter estimates for total plasma carotenoids from total cholesterol-adjusted, class-adjusted and overall multivariate-adjusted linear regression models*
(Parameter estimates and 95 % confidence intervals)

LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1.
* For details of procedures and subjects, see Methods and Table 1.
† Each multivariate model controlled for total cholesterol and each variable listed within each variable class.
‡ Each multivariate model controlled for total cholesterol and every other variable listed.
Table 4 presents results from total cholesterol-adjusted, class-adjusted and multivariate-adjusted logistic regression models comparing those above v. below the median level of total plasma carotenoids. Among clinical variables, no variable was associated with increased levels of total plasma carotenoids in the overall multivariate-adjusted model. Among the lifestyle variables, the OR and 95 % CI were statistically significant in all three models for current smoking (OR 0·10 (95 % CI 0·03, 0·36), OR 0·13 (95 % CI 0·04, 0·45) and OR 0·21 (95 % CI 0·06, 0·77), respectively) and daily alcohol ingestion (OR 2·27 (95 % CI 1·32, 3·88), OR 1·98 (95 % CI 1·14, 3·45) and OR 2·46 (95 % CI 1·29, 4·67), respectively). No other lifestyle variables had a significant association in any model. Significant positive associations with total plasma carotenoids were found for each 100 mg/l difference in LDL-C and 10 μmol/l difference in α-tocopherol in the class-adjusted and overall multivariate-adjusted models only. However, the OR for each 100 mg/l difference in HDL-C was statistically significant in all three models (OR 1·17 (95 % CI 1·02, 1·35), OR 1·42 (95 % CI 1·17, 1·72) and OR 1·58 (95 % CI 1·26, 1·99), respectively). Including the use of a multivitamin in the overall multivariate model did not significantly alter these results (data not shown).
Table 4 Odds ratios for above v. below the median value (1·301 μmol/l) of total plasma carotenoids from total cholesterol-adjusted, class-adjusted and overall multivariate-adjusted logistic regression models*
(Odds ratios and 95 % confidence intervals)

LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1.
* For details of procedures and subjects, see Methods and Table 1.
† Each multivariate model controlled for total cholesterol and each variable listed within each variable class.
‡ Each multivariate model controlled for total cholesterol and every other variable listed.
We also compared men in the highest quartile of total plasma carotenoids with men in the lowest quartile using multivariate-adjusted logistic regression models. The only statistically significant OR were for 10 mmHg difference in systolic blood pressure (OR 0·66; 95 % CI 0·47, 0·93), daily alcohol ingestion (OR 11·38; 95 % CI 3·37, 38·45), weekly alcohol ingestion (OR 9·45; 95 % CI 2·45, 36·43), monthly alcohol ingestion (OR 4·57; 95 % CI 1·28, 16·28), 100 mg/l increase in total cholesterol (OR 0·54; 95 % CI 0·36, 0·80), 100 mg/l increase in LDL-C (OR 2·21; 95 % CI 1·43, 3·42), 100 mg/l increase in HDL-C (OR 2·41; 95 % CI 1·62, 3·61), 100 ng/ml increase in ICAM-1 (OR 0·53; 95 % CI 0·29, 0·95) and 10 μmol/l increase in α-tocopherol (OR 1·83; 95 % CI 1·39, 2·41). Including the use of a multivitamin in the overall multivariate model did not significantly alter these results (data not shown).
Discussion
In the present cross-sectional study examining potential determinants of total plasma carotenoids in middle-aged and older men, few lifestyle and clinical risk factors appear to be related to levels of total plasma carotenoids. Only current smoking and daily alcohol ingestion appeared to be significantly associated with total plasma carotenoid levels. On the other hand, plasma lipids and α-tocopherol were strongly associated with total plasma carotenoid levels. More novel inflammatory biomarkers such as CRP, ICAM-1 and IL-6 were not associated with total plasma carotenoids when considered simultaneously with lipids and tocopherols. Of the individual carotenoids, levels of β-carotene and α-carotene correlated the most with levels of total plasma carotenoids.
The noted inverse correlation between smoking and levels of total plasma carotenoid has been observed in other studies(Reference Wallström, Wirfält, Lahmann, Gullberg, Janzon and Berglund14, Reference Wei, Kim and Boudreau15). Data from the ‘β-Carotene and Retinol Efficacy Trial’ (CARET) showed that for every ten cigarettes smoked per d, serum β-carotene concentration was 5·4 % lower(Reference Goodman, Thornquist, Kestlin, Metch, Anderson and Omenn16). Proposed mechanisms for the inverse relationship between total plasma carotenoid levels and cigarette smoking include decreased absorption of dietary carotenoids among active smokers and increased clearance of plasma carotenoids as a consequence of their interaction with free radicals produced as a result of cigarette smoking(Reference Northrop-Clewes and Thurnham17).
Dietary characteristics may partially explain the observed associations among HDL-C, α-tocopherol and total plasma carotenoids. Studies have shown that diets high in unsaturated fat may increase the absorption of individual and total carotenoids(Reference Ahuja, Pittaway and Ball18–Reference Lopez-Garcia, Schulze, Fung, Meigs, Rifai, Manson and Hu20). Dietary sources of unsaturated fats also tend to be sources of tocopherols. In addition, diets high in unsaturated fats are associated with increased levels of plasma HDL-C(Reference Sacks and Katan21). Another possible explanation for the relationship between carotenoids and tocopherols is that physicians may be more inclined to take multivitamin supplements that include both carotenoids and tocopherols. Unfortunately, we did not have information on the components of individual diets or multivitamin supplements for this analysis.
Previous studies have shown an inverse correlation between inflammatory markers and levels of carotenoids(Reference Blum, Aviram, Ben-Amotz and Levy19, Reference Lopez-Garcia, Schulze, Fung, Meigs, Rifai, Manson and Hu20, Reference Gruber, Chappell, Millen, LaRowe, Moeller, Iannaccone, Kritchevsky and Mares22, Reference van Herpen-Broekmans, Klopping-Ketelaars, Bots, Kluft, Princen, Hendriks, Tijburg, van Poppel and Kardinaal23). The Mediterranean diet may also be associated with increased serum levels of carotenoids and decreased serum levels of inflammatory markers such as CRP(Reference Blum, Aviram, Ben-Amotz and Levy19, Reference Lopez-Garcia, Schulze, Fung, Meigs, Rifai, Manson and Hu20). One study found a statistically significant inverse correlation between serum levels of ICAM-1 and both lutein and lycopene as well as between CRP and β-carotene(Reference van Herpen-Broekmans, Klopping-Ketelaars, Bots, Kluft, Princen, Hendriks, Tijburg, van Poppel and Kardinaal23). In our analysis, serum levels of each inflammatory marker decreased with increasing levels of total plasma carotenoids. However, in multivariate analyses, we did not find CRP, IL-6 or ICAM-1 to be statistically significant predictors for levels of total plasma carotenoids when we simultaneously considered other biomarkers such as lipids and tocopherols.
The present cross-sectional study examines plasma carotenoids with a comprehensive assortment of variables, but some potentially important limitations remain. First, this analysis is cross-sectional from which no causal relationships can be directly assessed. Second, we did not have specific information on diet or supplements. Third, we did not have information regarding the number of cigarettes consumed or the amount of alcohol consumed by these men. Though we relied upon self-reports from our physician participants, healthcare providers have been shown to be accurate reporters of their health status(Reference Colditz, Martin, Stampfer, Willett, Sampson, Rosner, Hennekens and Speizer24). Finally, our analyses were limited to predominantly white, apparently healthy, middle-aged and older male physicians and therefore may not apply to women, men with poor health, younger men, or men at other socioeconomic levels. There may be differences in plasma carotenoids according to sex, ethnic and cultural groups(Reference Brady, Mares-Perlman, Bowen and Stacewicz-Sapuntzakis4–Reference Rock, Thornquist, Neuhouser, Kristal, Neumark-Sztainer, Cooper, Patterson and Cheskin7) that warrant additional research.
In conclusion, total plasma carotenoid levels were primarily influenced by smoking, alcohol ingestion, lipid parameters and α-tocopherol. We found that current smoking was associated with lower levels of total plasma carotenoids, while daily alcohol ingestion, increasing plasma levels of LDL-C, HDL-C and α-tocopherol were each significantly associated with increasing levels of total plasma carotenoids. Understanding the predictors of total plasma carotenoids may improve our understanding of risk factors for chronic conditions such as CVD and cancer.
Acknowledgements
The present study was funded by NIH CA 97193 and BASF AG, plus a grant from Roche Vitamins, Inc. Approximately half of the participants in PHS II also participated in PHS I, which was established through NIH CA 34944, CA 40360, HL 26490 and HL 34595. Work on the present study was also supported by the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development.
This research was supported in part by a grant from Roche Vitamins, Inc. The authors have no other financial or personal interests related to this research. The authors would like to acknowledge the crucial contributions of the entire staff of the PHS. We are also indebted to the 29 071 dedicated and committed participants randomised into the PHS starting in either 1982 or 1995.






