Adequate iodine intake is essential for fetal and postnatal development. Iodine deficiency during pregnancy may result in in utero hypothyroidism and increase the rates of miscarriage, stillbirths, as well as congenital abnormalities such as cretinism, a grave irreversible form of mental retardation (Glinoer, Reference Glinoer1997; Utiger, Reference Utiger1999). The goal of eliminating iodine-deficiency disease has been achieved since universal salt iodisation policy has been widely carried out in many nations including China (Delange & Lecomte, Reference Delange and Lecomte2000). On the other hand, reports are increasingly appearing on the toxic effects caused by high amounts of iodine intake. Exposure to excessive iodine occurs via food (Konno et al. Reference Konno, Makita, Yuri, Iizuka, Kawasaki, Norimichi, Horini, Kenji, Norio and Kimio1994), drinking water (Zhao et al. Reference Zhao, Chen and Maberly1998), medication (Martino et al. Reference Martino, Bartalena, Bogazzi and Braverman2001) and iodised salt or iodinated oil (Wolff, Reference Wolff2001). Recent studies have reported that iodine excess also causes either hypothyroidism or hyperthyroidism (Markou et al. Reference Markou, Georgopoulos, Kyriazopoulou and Vagenakis2001; Roti & Uberti, Reference Roti and Uberti2001), which may induce embryo toxicity, especially skeletal anomalies. Though little is known about the cellular and molecular basis for these abnormalities, disruption of thyroid hormone metabolism and modulation of the expression pattern of genes involved in differentiation, growth and metabolism mediated by thyroid hormones may play a pivotal role in this process.
Excessive iodine has a complex disruptive effect on thyroid hormone metabolism. Animal studies (Bednarczuk et al. Reference Bednarczuk, Pietrzykowski, Slon and Nauman1993) have suggested that excessive iodine-induced thyroid hormone abnormalities are related to the inhibition of the activity of type 1 deiodinase (D1), which catalyses the deiodination of both the outer and inner rings of thyroxine (T4) and is responsible for most of the circulating triiodothyronine (T3) (Bianco et al. Reference Bianco, Salvatore, Gereben, Berry and Larsen2002). Fetal thyroid hormones must come from the maternal circulation before the fetal thyroid gland and pituitary–thyroid axis become functional (Obregon et al. Reference Obregon, Mallol, Pastor, Morreale de Escobar and Escobar del Rey1984). The maternal transfer of T4 constitutes a major fraction of fetal thyroid hormones, even after the onset of fetal thyroid secretion (Morreale de Escobar et al. Reference Morreale de Escobar, Pastor, Obregon and Escobar del Rey1985; Burrow et al. Reference Burrow, Fisher and Larsen1994; Santini et al. Reference Santini, Chiovato and Ghirri1999). Therefore, the changes of maternal thyroid hormone level have an affirmative effect on the fetus by deiodination of iodothyronines through the placenta. Placental type 2 deiodinase (D2), which mainly catalyses the outer ring deiodination of T4, and type 3 deiodinase (D3), which inactivates T4 and T3, may have an important function in regulating fetal thyroid hormone levels. However, the effect of maternal excessive iodine exposure on placental D2 and D3 activity has not been reported.
Hox genes, namely homeobox-containing genes, are a cluster of genes which encode transcriptional factors regulating many aspects of development. Expression patterns of Hox genes are characterised by spatial collinearity, temporal collinearity and retinoic acid sensitivity collinearity (Lufkin, Reference Lufkin1996; Martinez & Amemiya, Reference Martinez and Amemiya2002). Thyroid hormone receptor (TR) and retinoic acid receptor have been shown to share an identical P-box sequence, which implicates that they can bind the same DNA sequences and can interact physically (Kumar & Thompson, Reference Kumar and Thompson1999). In frog embryogenesis, TR can modulate retinoic acid-mediated axis formation, and small changes in levels of TR in early embryos may directly affect the retinoic acid responsiveness of Xhox.lab2. In addition, PCR assays have shown that T3 can induce the expression of Xhox.lab2 in embryos which ectopically expressed TRα (Banker & Eisenman, Reference Banker and Eisenman1993). However, little information is available in the literature on Hox genes expression regulated directly by thyroid hormones in mammals. The structural analysis of Hox3.1 (Hoxc8) transcription unit and the Hox3.2-Hox3.1 intergenic region found that there is a thyroid hormone response element in the transcriptional regulation region of the mouse Hoxc8 gene (Awgulewitsch et al. Reference Awgulewitsch, Bieberich, Bogarad, Shashikant and Ruddle1990), which implicated that thyroid hormones may regulate Hoxc8 expression during mouse embryogenesis.
Hoxc8 belongs to the Hox gene family and expresses in limbs, backbone rudiments, the neural tube of mouse mid-gestation embryos, and in the cartilage and skeleton of newborns (Kwon et al. Reference Kwon, Shin, Park and Kim2005). Skeletal abnormalities in ribs, sternum and vertebrae have been observed in Hoxc8 knockout mice (Akker et al. Reference Akker, Fromental-Ramain, Graaff, Mouellic, Brûlet, Chambon and Deschamps2001; Juan & Ruddle, Reference Juan and Ruddle2003). These findings suggested that Hoxc8 is an important regulator of pattern formation during the development of the vertebrate skeleton. Our laboratory has previously illustrated (Yang et al. Reference Yang, Xu, Hou, Guo, Hao, Yao, Liu and Sun2006) that maternal excessive iodine exposure resulted in defects in skeletal patterning in fetuses, such as supernumerary ribs, agenesis of sternbrae, poor ossification of metacarpals and metatarsals and distortion of vertebrae. Such alternations induced by excessive iodine may be related to the modulation of Hoxc8 expression by thyroid hormones. However, little in the literature was available on this hypothesis. Therefore, we conducted the present study to determine whether maternal and fetal thyroid hormone metabolism was influenced, and whether Hoxc8 expression pattern was regulated by excessive iodine exposure during mouse embryogenesis.
Materials and methods
Animals and treatment
Weaning Balb/C mice obtained from the Laboratory Animal Centre of Hubei Provincial Centre for Disease Control and Prevention (Wuhan, China) were maintained in constant temperature-controlled rooms (22 ± 2°C) with controlled lighting (12 h light–dark cycle). All animals were housed in stainless steel cages and given a commercial laboratory chow and sterile water ad libitum. The content of iodine in the diet and water was 365 μg/kg and 8 μg/l respectively. The animals were cared for according to the Guiding Principles in the Care and Use of Animals. The experiments were approved by the Tongji Medical College Council on Animal Care Committee.
After acclimatisation to the laboratory environment for 1 week, animals were randomly assigned to six groups of twelve animals each (eight females and four males) according to body weight and given different doses of iodine in the form of potassium iodate (KIO3) in the drinking water at the levels of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml by using sterile water as the vehicle. Water consumption of each group was recorded. Female mice were placed into the metabolism cages, 4 months later, of four mice each and urine samples of 3 h in the morning were collected for 3 d for urinary iodine concentration determination. Then females were paired with a male in a 2:1 ratio overnight and examined for the presence of a vaginal plug in the following morning, which was defined as 0·5 days postcoitum (dpc). The treatment with high doses of iodine continued through the period of gestation. Dams were killed by cervical dislocation on 12·5 dpc and blood was collected for thyroid hormone analysis. Placentas were collected immediately, frozen in liquid N2 and stored at − 80°C for D2 and D3 activity determination. Embryos were dissected free of the maternal and extra-embryonic tissue in PBS, then frozen in liquid N2 and stored at − 80°C for RT-PCR and Western analysis.
Iodine concentration and thyroid hormone analysis
Iodine concentration in diet, water and urine was measured by the Cer-Arsenite colorimetric method as modified by Fischer et al. (Reference Fischer, L'Abbé and Giroux1986). Urinary creatinine concentrations were determined by the alkaline picrate method. The urinary iodine:creatinine ratio (μg/g Cr) was used to estimate urinary iodine concentration. Serum total T4 and total T3 were measured by RIA kits obtained from the Chinese Academy of Atomic Energy (Beijing, China).
Hepatic and renal type 1 deiodinase activity assays
Tissues were homogenised in cold hydroxyethyl piperazine ethanesulfonic acid buffer solution (dithiothreitol (1 mmol/l), hydroxyethyl piperazine ethanesulfonic acid buffer (10 mmol/l), pH 7·0, sucrose (320 mmol/l)) at a 1:39 and 1:24 dilution (w/v) for livers and kidneys, respectively. Homogenates were centrifuged at 1500 g for 10 min at 4°C. The supernatant fraction was re-centrifuged at 20 000 g for 5 min at 4°C, floating debris removed and supernatant fraction used for D1 assay.
D1 activity was measured using [125I]5′-rT3 (0·005 μmol [125I]5-rT3/l, 1000 μCi/μg; Northern Biotechnology Ltd, Beijing, China; and 0·495 μmol 5′-l-rT3/l; Sigma, St Louis, MO, USA) as substrate and in the presence of dithiothreitol (2 mmol/l), EDTA (1 mmol/l) and potassium phosphate buffer (100 mmol/l), pH 7·0, based on methods previously described (Hotz et al. Reference Hotz, Belonje Bitzpatrick and L'abbe1996). Enzyme activity was expressed as pmol of I− released/mg protein per min of reaction. Protein concentrations were determined using the method of Bradford (Reference Bradford1976).
Placental type 2 and type 3 deiodinase activity assays
Placentas were homogenised at a 1:4 (w/v) dilution in hydroxyethyl piperazine ethanesulfonic acid (10 mmol/l; pH 7·2), sucrose (250 mmol/l) and dithiothreitol (10 mmol/l). Homogenates were stored at − 80°C until further use. The measurement of D3 and D2 specific enzyme activities were performed as described previously (Koopdonk-Kool et al. Reference Koopdonk-Kool, de Vijlder, Veenboer, Ris-stalpers, Kok, Vulsma, Boer and Visser1996). In short, D3 activity was determined using [125I]5-T3 (0·6 nmol [125I]5-T3/l, 2000 μCi/μg; Northern Biotechnology Ltd, Beijing, China; and 1 nmol T3/l; Sigma) as substrate, measured by the amount of I− released in the conversion of [125I]5-T3 to diiodotyrosine by placental homogenates, and was corrected for non-enzymic 5-deiodination. D2 activity was determined using [125I]5′-T4 (0·3 nmol [125I]5′-T4/l, 2000 μCi/μg; Northern Biotechnology Ltd; and 1 nmol T4/l; Sigma) as substrate, measured by the amount of I− released in the conversion of [125I]5′-T4 to T3 and also corrected for non-enzymic deiodination; further deiodination was inhibited by adding excess non-radioactive T3 (Sigma). Enzyme activities were expressed as fmol of 125I− released from [125I]5′-T4 (D2) or [125I]5-T3 (D3)/h per mg protein. Protein concentrations were determined using the method of Bradford (Reference Bradford1976), with bovine serum albumin as standard.
Semi-quantitative reverse transcriptase- polymerase chain reaction assay
Total RNA of 12·5 d embryos was extracted by TriZol reagent (Gibco, Grand Island, NY, USA). RNA (2 μg) was reverse-transcribed with random hexamers by Moloney murine leukaemia virus RT, and then PCR were carried out using the following primers: Hoxc8 5′-GTCCAAGACTTCTTCCACCA-3′ (sense); 5′-CCTTGTCCTTCGCTACTGTT-3′ (antisense) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 5′-TCACTCAAGATTGTCAGCAA-3′ (sense); 5′-AGATCCACGACGGACACATT-3′ (antisense) generating products of 215 and 308 bp respectively. All PCR reactions consisted of dNTP (0·2 mmol/l), 2 μl cDNA, 0·25 μmol/l of each primer, 1 × PCR buffer and 0·8 U Taq polymerase. The following cycling profile was used: 5 min of denaturation at 94°C followed by thirty-five cycles of each 1 min at 94°C denaturation, 1 min of annealing (GAPDH 58°C and Hoxc8 57°C) and 1 min extension at 72°C, and a final extension step of 10 min at 72°C in a PCR System thermocycler (Whatman, Biometra, Germany). The PCR products were separated on 1·5 % agarose gel and stained with ethidium bromide. Quantification of the Hoxc8 and GAPDH mRNA was performed by scanning the intensities of the ethidium bromide-stained PCR products using the BioDocAnalyse system (Whatman). The Hoxc8 mRNA levels were standardised relative to GAPDH mRNA.
Western analysis
Extraction nuclear protein from 12·5 dpc embryos was performed as described previously. The protein concentration was determined by Dc protein assay (BioRad, Richmond, CA, USA). Nuclear protein samples (50 μg) were heated for 5 min at 95°C and separated on 12 % SDS-PAGE and transferred to NC membranes (Millipore, Bedford, MA, USA) in tri(hydroxymethyl)-aminomethane-glycine buffer(pH 8·5) plus 20 % methanol. The membranes were blocked overnight in 5 % non-fat milk in tri(hydroxymethyl)-aminomethane-buffer containing 0·1 % Tween-20 and then washed with tri(hydroxymethyl)-aminomethane-buffer. The blots were incubated for 2 h at room temperature with 1:500 mouse Hoxc8 monoclonal IgG (Covance, Princetown, NJ, USA) and 1:4000 rabbit polyclonal antibody anti-nucleolin (Abcam Ltd, Cambridge, Cambs, UK), respectively. The blots were washed and then incubated with anti-mouse IgG conjugated with peroxidase (Sigma, St Louis, MO, USA) at 1:10 000 dilution. An Amersham ECLTM Detection Kit (GE Healthcare Life Sciences, Little Chalfont, Bucks, UK) provided the chemiluminescence substrate for horseradish peroxidase, and the targeted protein was visualised by autoradiography.
Statistical methods
The SPSS 12·0 software package (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Because of its skewed distribution, the medians were used to describe the central tendency of urinary iodine concentration. The Kruskal–Wallis method was used to test the differences in ranking of urinary iodine concentration. Other data were analysed by a one-way ANOVA and Duncan's test. Significance level was set at P < 0·05.
Results
Average daily water consumption, urinary iodine concentration and thyroid hormone level in maternal mice
Average daily water intake was 4·9 (sd 0·8), 4·8 (sd 0·9), 4·8 (sd 1·2), 4·5 (sd 1·3), 4·2 (sd 0·7) and 4·2 (sd 1·2) ml in female mice of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg iodine/ml groups, respectively. There was no obvious difference among groups. The mouse drinks about 5 ml daily. As for the groups given high doses of iodine, iodinated water was the main source of iodine. So, the daily iodine intake could be about 7·5, 15, 30, 60 and 120 μg in the treatment groups, which corresponded to 5-, 10-, 20-, 40- and 80-fold of the adequate iodine intake for mice. The concentration of iodine in urine is currently the most widely used biochemical marker of iodine intake. After exposure to excessive iodine for 4 months, the urinary iodine concentration of female mice increased in a dose-dependent manner (r 0·96; P < 0·01; Fig. 1). Compared with the control group, serum total T4 levels increased and serum total T3 levels decreased significantly in dams when the iodine dose reached 3·0 μg/ml, whereas exposure to 1·5 μg iodine/ml had no obvious effect on thyroid hormone level (Fig. 2).
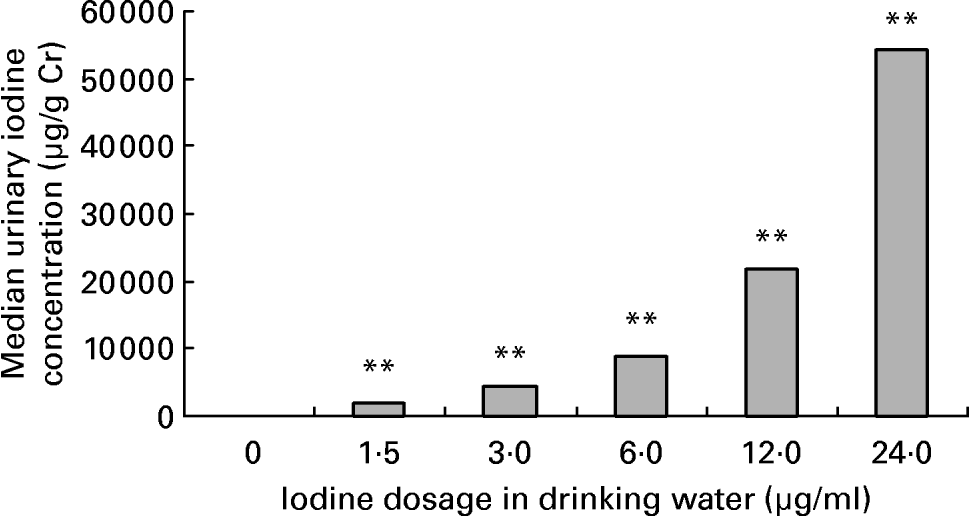
Fig. 1 Effect of excessive iodine exposure on urinary iodine level in female mice. Exposed to different doses of iodine at the levels of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml in drinking water for 4 months, female mice were placed into metabolism cages of four mice each and urine samples of 3 h in the morning were collected for 3 d for urinary iodine determination. The urinary iodine:creatinine ratio (μg/g Cr) was used to estimate iodine concentration in urine. Values are medians, each bar representing the median of a group of six samples. ** Median values were significantly different from that of the control group (P < 0·01) (Kruskal–Wallis method).
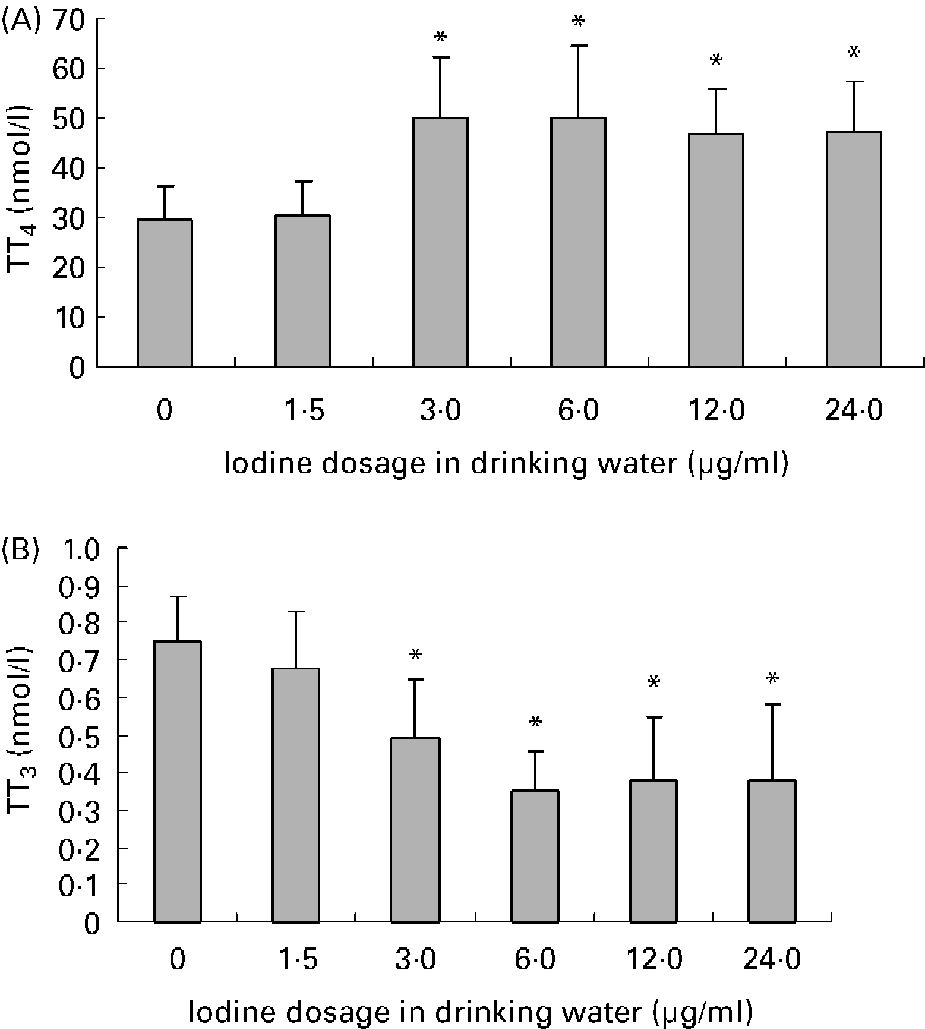
Fig. 2 Effects of excessive iodine exposure on serum thyroid hormone levels in maternal mice. Serum was collected from 12·5 d postcoitum maternal mice exposed to different doses of iodine at the levels of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml in drinking water. Values are means for serum total thyroxine (TT4) level (A) and total triiodothyronine (TT3) level (B) (n 8), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P < 0·05) (ANOVA and Duncan's test).
Hepatic and renal type 1 deiodinase activity, and placental type 2 and type 3 deiodinase activity assays
An obvious depression of D1 activity in liver and kidney was observed in groups when the exposure iodine dose reached 3·0 μg/ml; this showed in a dose-dependent manner (liver: r − 0·402, P < 0·01; kidney: r − 0·276, P < 0·05; Fig. 3). High iodine intake had a predominant effect on D2 activity of 12·5 dpc placenta, and no effect on D3 activity (Fig. 4). A dose-dependent reduction of D2 activity was found in groups where the dose was 3·0 μg/ml or above (r − 0·524; P < 0·01). Meanwhile, D3 activity was obvious higher than D2 activity in 12·5 dpc placenta.

Fig. 3 Effect of excessive iodine exposure on type 1 deiodinase (D1) activity in liver (A) and kidney (B) of maternal mice. Exposed to different doses of iodine at the levels of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml in drinking water for 4 months, female mice were mated and killed at 12·5 d postcoitum. D1 activities of liver and kidney were determined using [125I]r-triiodothyronine as substrate. Enzyme activity was expressed as pmol I− released/mg protein per min of reaction. Values are means for D1 activity in liver (A) and kidney (B) (n 8), with standard deviations represented by vertical bars.* Mean value was significantly different from that of the control group (P < 0·05) (ANOVA).

Fig. 4 Effect of excessive iodine exposure on placenta type 2 deiodinase (D2) (A) and type 3 deiodinase (D3) (B) activities at 12·5 d postcoitum. Exposed to different doses of iodine at the levels of 0, 1·.5, 3·0, 6·0, 12·0 and 24·0 μg/ml in drinking water for 4 months, female mice were mated and killed at 12·5 d postcoitum. Placental D2 and D3 activities were determined using [125I]thyroxine (T4) and [125I] triiodothyronine (T3) as substrate, respectively. Enzyme activities were expressed as fmol 125I− released from [125I]T4 (D2) or [125I]T3 (D3)/h per mg protein. Values are means for placental D2 (A) and D3 (B) activities (n 8), with standard deviations represented by vertical bars. ** Mean value was significantly different from that of the control group (P < 0·01) (ANOVA).
Hoxc8 messenger ribonucleic acid and protein expression
In the case of the temporal expression pattern, Hoxc8 was expressed in most of the stages of embryonic development from 8·5 to 17·5 dpc (Kwon et al. Reference Kwon, Shin, Park and Kim2005). In the present study, a decreasing trend in mRNA abundance was semi-quantified by RT-PCR in 12·5 dpc embryos exposed to excessive iodine (Fig. 5 (A)). Western blot assay indicated that high iodine intake above 1·5 μg/ml induced down regulation of Hoxc8 protein (Fig. 5 (B)).

Fig. 5 Effect of excessive iodine exposure on mRNA and protein expressions of Hoxc8 in 12·5 d postcoitum embryos. Exposed to different doses of iodine at the levels of 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml in drinking water for 4 months, female mice were mated and killed at 12·5 d postcoitum. Embryos were collected, and RT-PCR and Western blotting were performed to determining mRNA and protein expressions of Hoxc8. PCR products were visualised by ethidium bromide staining (A). Protein expression was quantified by Western analysis with ECL detection (B) (see Methods). Lane M, DNA marker; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; lanes 1, 2, 3, 4, 5 and 6 represent embryos from groups receiving iodine at 0, 1·5, 3·0, 6·0, 12·0 and 24·0 μg/ml.
Discussion
In the present study, excessive iodine treatment resulted in an increase of total T4 and a decrease of total T3, consistent with previous studies (Harjai & Licata, Reference Harjai and Licata1997; Xiang et al. Reference Xiang, Chen, Di, Yan and Chen1999). This change was mainly related to the inhibition of D1 activity in liver and kidney, resulting in a decrease in the generation of T3 from T4. Maternal thyroid hormone levels have an effect on fetal thyroid hormones by means of the placenta, which modulates the transfer of iodine and small but important amounts of thyroid hormones (especially T4) from the mother to the fetus (Burrow et al. Reference Burrow, Fisher and Larsen1994). The fetal thyroid gland becomes functional at about 17–18 dpc in rodents (Bianco et al. Reference Bianco, Salvatore, Gereben, Berry and Larsen2002). At 12·5 dpc, fetal thyroid hormones come from the maternal circulation by deiodination of T4 through placenta D2. Placental D2 activity is negatively regulated by maternal T4 level (Steinsapir et al. Reference Steinsapir, Bianco, Buettner, Harney and Larsen2000). In the present study, placental D2 activity at 12·5 dpc decreased, showing an inverse relationship with maternal T4 level (r − 0·301; P < 0·05). Placental D2 activity is likely to be of considerable physiological importance for fetal thyroid hormone economy by contributing to the intraplacental T3 content, and possibly to the plasma T3. Placental D3 activity is much higher than that of D2, which could be important for the protection of fetal tissues from elevated T3 levels (Bates et al. Reference Bates, St Germain and Galton1999). Placental D3 activity showed no significant change after exposure to excessive iodine; the underlying mechanism needs to be elucidated. At 12·5 dpc, the mother is the only source of fetal thyroid hormones. After exposure to excessive iodine, T4 was higher but T3 was lower in the serum of maternal mice. At the same time, placental D2 activity decreased. Therefore, thyroid hormone abnormalities – either hypothyroidism or hyperthyroidism – may be induced in 12·5 d embryos. Due to limited sample availability, thyroid hormone levels in the fetuses were not measured in the present study. Nevertheless, several studies have reported that chronic maternal exposure to excessive iodine may cause fetal or neonatal hypothyroidism and goitre (Mehta et al. Reference Mehta, Mehta and Vorherr1983; Bartalena et al. Reference Bartalena, Bogazzi and Braverman2001; Serreaul et al. Reference Serreaul, Polack and Leger2004). In addition, another study (Guo et al. Reference Guo, Xu, Yang, Hou, Chen and Sun2006) in our laboratory also found that the progenies of mothers exposed to excessive iodine were hypothyroid. The above evidence has suggested that hypothyroidism might be induced in 12·5 d embryos of mothers exposed to excessive iodine.
Thyroid hormones are essential for normal skeletal development (Yen, Reference Yen2001). Regulation of T3 on chondrocytes, osteoblasts and osteoclasts, and the actions of TR isoforms in skeletal development has been reviewed (Bassette & Williams, Reference Bassette and Williams2003). Hypothyroidism may result in growth arrest, delayed bone age and short stature (Yen, Reference Yen2001). We previously observed that excessive iodine exposure increased the incidence of skeletal malformation, especially supernumerary ribs. A similar phenomenon was observed in Hoxc8-/- mice (Akker et al. Reference Akker, Fromental-Ramain, Graaff, Mouellic, Brûlet, Chambon and Deschamps2001). Other several lines of evidence also substantiate a role for Hoxc8 in the normal axial skeleton (Belting et al. Reference Belting, Shashikant and Ruddle1998; Juan & Ruddle, Reference Juan and Ruddle2003; Kwon et al. Reference Kwon, Shin, Park and Kim2005). In the present study, maternal excessive iodine exposure down regulated mRNA and protein expression of Hoxc8 in 12·5 d embryos. Gaur et al. (Reference Gaur, Zajdel, Bhatia, Isitmangil, Denz, Robertson, Lemanski and Dube2001) described a dramatic increase in the expression of the HoxA5 in the heart and aorta of the Mexican axolotl during the process of T4-induced metamorphosis. Disruption of Hoxc8 expression may associate with the fluctuation of thyroid hormone level induced by excessive iodine exposure. Moreover, with a thyroid hormone response element located in the Hoxc8 promoter region, hypothyroidism induced by excessive iodine could reduce Hoxc8 expression through this thyroid hormone response element-dependent pathway. This finding provided a possible explanation for the skeletal malformation induced by excessive exposure. Further studies are needed to provide more direct evidence of Hoxc8 expression regulation by thyroid hormones through this thyroid hormone response element-dependent pathway.
The mechanism of Hoxc8 modulating bone development has not been clarified. Recently, the identification of downstream targets of Hoxc8 genes found that osteopontin (OPN), also known as secreted phosphoprotein 1, is down regulated by Hoxc8 overexpression in microarray analysis and confirmed by chromatin immunoprecipitation (ChIP) analysis (Lei et al. Reference Lei, Wang, Juan and Ruddle2005). OPN is the major non-collagenous bone matrix protein associated with osteoblastic cell adhesion and abundantly expressed during the early stages of osteoblast differentiation. More interestingly, analysis of thyroid hormone responsive gene expression found that OPN expression is also regulated by T3 in osteoblastic cells (Harvey et al. Reference Harvey, Stevens, Williams, Jackson, O'Shea and Williams2003). These findings provide more possible evidence of bone development modulated by thyroid hormones through Hox genes, which also need further investigation to verify.
In conclusion, we have demonstrated that excessive iodine exposure induced abnormalities of maternal–fetal thyroid hormone metabolism by affecting deiodinase activities, accompanying down regulation of Hoxc8 mRNA and protein expression. This mechanism may play a pivotal role in skeletal malformation induced by excessive iodine, and provide a new clue to study the relationship between nutrient–iodine or thyroid hormones and Hox gene expression pattern.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China, no. 30230330.







