Dietary advice to replace saturated fat with PUFA to reduce the incidence of CVD(Reference Harris, Mozaffarian and Rimm1, Reference Mozaffarian, Micha and Wallace2), and increasing use of vegetable oils, have led to a dramatic increase in the human consumption of linoleic acid (LA, 18 : 2n-6) during the twentieth century(Reference Blasbalg, Hibbeln and Ramsden3). The estimated per capita consumption of soyabean oil, one of the major dietary sources of LA in the USA, has increased more than 1000-fold from 1909 to 1999, increasing the availability of LA from 2·8 to 7·2 % of energy (en%)(Reference Blasbalg, Hibbeln and Ramsden3). Dietary intakes of n-3 and n-6 fatty acids are critical determinants of tissue proportions of bioactive 20- and 22-carbon n-3 and n-6 highly unsaturated fatty acids. The tissue fatty acid composition reflects dietary PUFA intake since 18-carbon n-3 and n-6 fatty acids cannot be synthesised de novo (Reference Lands, Libelt and Morris4). The endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide (AEA) are endogenous lipid mediators formed from the pool of arachidonic acid (AA) in membrane phospholipids (AA-PL). Hence, endocannabinoid activity can be altered by dietary fatty acids that in turn affect endocannabinoid precursor levels after a short term(Reference Artmann, Petersen and Hellgren5, Reference Wood, Williams and Pandarinathan6) or prolonged feeding(Reference Matias, Petrosino and Racioppi7–Reference Alvheim, Malde and Osei-Hyiaman10). Dietary LA(Reference Alvheim, Malde and Osei-Hyiaman10) and AA(Reference Artmann, Petersen and Hellgren5, Reference Matias, Petrosino and Racioppi7, Reference Berger, Crozier and Bisogno11) increased, whereas dietary n-3 PUFA(Reference Artmann, Petersen and Hellgren5, Reference Wood, Williams and Pandarinathan6, Reference Alvheim, Malde and Osei-Hyiaman10, Reference Di Marzo, Griinari and Carta12–Reference Batetta, Griinari and Carta15), decreased 2-AG and AEA. The activation of the cannabinoid receptor 1 by endocannabinoids or exogenous agonists, centrally and peripherally, favours metabolic processes that stimulate appetite, increase food intake, activate fat storage pathways and down-regulate catabolism resulting in adipose accumulation(Reference Osei-Hyiaman, DePetrillo and Pacher8, Reference Alvheim, Malde and Osei-Hyiaman10, Reference Cota, Marsicano and Tschop16).
The type of fat, specifically the imbalance in n-6 to n-3 PUFA, is emerging as a risk factor for developing obesity(Reference Ailhaud, Guesnet and Cunnane17–Reference Matias, Carta and Murru20, Reference Matias, Petrosino and Racioppi7). Dietary LA have been shown to have adipogenic properties in both humans(Reference Dayton, Hashimoto and Dixon21) and rodents(Reference Madsen, Pedersen and Liaset18, Reference Massiera, Saint-Marc and Seydoux19, Reference Cunnane, Manku and Horrobin22–Reference Okuno, Kajiwara and Imai25). Fish oils rich in EPA and DHA limit diet-induced obesity in rodents(Reference Belzung, Raclot and Groscolas26, Reference Hill, Peters and Lin27) and are associated with weight reduction in humans(Reference Kunesova, Braunerova and Hlavaty28–Reference Thorsdottir, Tomasson and Gunnarsdottir30). We have previously shown a robust positive correlation between the consumption of soyabean oil, the major contributor to dietary LA, and obesity in human subjects(Reference Alvheim, Malde and Osei-Hyiaman10). When investigating these human dietary changes over the last century in an animal model, we have demonstrated that it is the n-3 and n-6 fat composition of the diets, rather than the total amount of fat, that determined the obesogenic properties of a diet(Reference Alvheim, Malde and Osei-Hyiaman10). High-fat diets (60 en%) commonly used to induce obesity typically utilise soyabean oil and contain high levels of LA. Such diets elevate endocannabinoid levels in tissues involved in energy homeostasis contributing to diet-induced obesity in mice after long-term feeding(Reference Matias, Petrosino and Racioppi7, Reference Osei-Hyiaman, DePetrillo and Pacher8). We have recently reversed the obesogenic effect of high-fat isoenergetic diets by decreasing dietary LA from 8 to 1 en% and attenuated the AA-dependent excessive endocannabinoid activity(Reference Alvheim, Malde and Osei-Hyiaman10). Furthermore, we have demonstrated that reducing the AA-PL precursor pool by adding 1 en% EPA and DHA to 8 en% LA diets reversed both the stimulation of endocannabinoid activity and the obesogenic effect of high-fat diets(Reference Alvheim, Malde and Osei-Hyiaman10). Reducing excessive endocannabinoid system activity is actively being pursued to reduce obesity(Reference Osei-Hyiaman, DePetrillo and Pacher8). The pharmacological blockade of the cannabinoid receptor 1 is effective in treating obesity and related metabolic derangements(Reference Despres31, Reference Van Gaal, Rissanen and Scheen32). However, serious psychiatric side effects caused the withdrawal of rimonabant, a selective cannabinoid receptor 1 antagonist(Reference Christensen, Kristensen and Bartels33, Reference Christopoulou and Kiortsis34). Thus, there is a definite need for a dietary approach to reduce substrate availability for endocannabinoid synthesis.
The high degree of conservation of endocannabinoid system components(Reference Elphick and Egertova35) and the presence of the cannabinergic system in most animal systems(Reference De Petrocellis, Melck and Bisogno36–Reference Sepe, De Petrocellis and Montanaro40) point out the importance of the endocannabinoid system in the regulation of basic physiological responses such as energy homeostasis and feeding behaviour(Reference De Petrocellis, Melck and Bisogno36–Reference Valenti, Cottone and Martinez38, Reference Kirkham, Williams and Fezza41, Reference Piccinetti, Migliarini and Petrosino42). Farmed Atlantic salmon have traditionally been fed diets based on fish oil and fishmeal, thus being recognised as a rich source of the marine n-3 fatty acids EPA and DHA. The steady increase in aquaculture production volume of 8–10 % per year(Reference Tacon, Hasan and Subasinghe43) has resulted in the increased use of alternative proteins and oils in aqua feeds. Vegetable oils are recognised as suitable alternatives to fish oils(Reference Torstensen, Bell and Rosenlund44, Reference Turchini, Torstensen and Ng45), although they are devoid of EPA and DHA, with high levels of LA and monounsaturates, thereby reducing EPA and DHA and increasing LA content of fish fillet(Reference Torstensen, Bell and Rosenlund44–Reference Grisdale-Helland, Ruyter and Rosenlund46). The current fish oil replacement levels in aqua feeds are approximately 50 %, resulting in approximately 2 g EPA+DHA/100 g salmon flesh (www.nifes.no). This fish oil replacement level is expected to increase in future aquaculture feeds as Atlantic salmon production volumes increase(Reference Tacon, Hasan and Subasinghe43) whereas global availability of fish oil remains constant (www.iffo.net). However, there is a lack of documentation of the health consequences of replacing fish oil with vegetable oil in feed for Atlantic salmon in fish consumers. We here posit that excessive dietary LA elevates endocannabinoid activity in salmon and mice, with a concomitant increase in adiposity in mice fed LA-enriched salmon.
Methods
Feeding experiment in Atlantic salmon
Ethical statement
The experiments complied with the guidelines of the Norwegian Regulation on Animal Experimentation and EC Directive 86/609/EEC. The protocol was approved by local authorities at the Institute of Marine Research (Bergen, Norway) and the National Animal Research Authority.
Animals
Atlantic salmon (initial weight 340 (sem 17) g (n 55)) were randomly distributed to six fibreglass tanks (1·5 × 1·5 × 0·9 m, water depth 0·6 m) provided with a continuous flow of seawater maintaining an average salinity of 35‰, an average temperature 8°C and a 12 h light–dark cycle. Mortality was recorded daily. The feeding trial was carried out at the Matre Aquaculture Research Station (Matredal, Norway; 60°52′N, 05°35′E) from 28 April 2008 to 6 October 2008.
Diets
Experimental diets (EWOS Innovation) provided as pellets were fed ad libitum to triplicate tanks per dietary treatment for 6 months. The diets contained the same 6 mm base pellet and were supplemented with either cleaned fish oil (FO; FF Skagen) or refined soyabean oil (SO; Mills) (Table 1) to ensure negligible amounts of contaminants such as persistent organic pollutants and polyaromatic hydrocarbons.
Table 1 Nutrient composition of mice and salmon diets
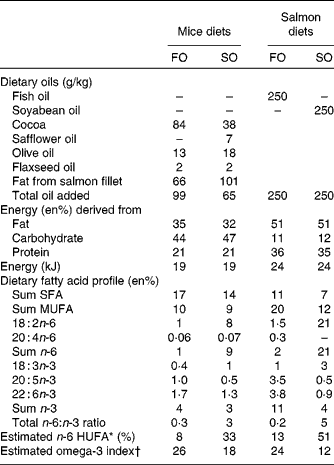
FO, fish oil; SO soyabean oil; en%, percentage of energy; HUFA, highly unsaturated fatty acids.
* Calculated from the Lands equation(Reference Lands, Libelt and Morris4): (20 : 3n-6+20 : 4n-6+22 : 5n-6)/(20 : 3n-6+20 : 4n-6+22 : 5n-6+20 : 5n-3+22 : 5n-3+22 : 6n-3) × 100.
† Calculated from (n-3 HUFA × 0·32) − 3·5 based on Harris & von Schacky(Reference Harris and von Schacky69) and Lands(Reference Lands70).
Sampling
Fish were feed-deprived 24 h before killing. From each tank, six fish were randomly sampled and killed with a sharp blow to the head to ensure no contamination of anaesthetics in the fish fillet. Body weight and length were measured, blood was collected and liver was quickly snap-frozen in liquid N2 and stored at − 80°C until further analysis. The fillets were collected for use in mice feed.
Lipid extraction and fatty acid analysis
Total lipid was extracted from the Atlantic salmon diets, liver and fillets by homogenisation in chloroform–methanol (2:1, v/v) with 19 : 0 methyl ester as the internal standard. Fatty acid methyl esters (FAME) were prepared from total lipid by BF3 following saponification, as described previously(Reference Lie and Lambertsen47, Reference Torstensen, Nanton and Olsvik48). Lipid classes of salmon liver were determined essentially as described by Jordal et al. (Reference Jordal, Lie and Torstensen49) based on Bell et al. (Reference Bell, Dick and Mc Vicar50).
Feeding experiment in C57BL/6J mice
Ethical statement
The mouse experiment was approved by the National Animal Health Authorities (Norwegian approval identification 1973). Care and handling were in accordance with local institutional recommendations and rules, and no adverse events were observed. Mice were anaesthetised with isoflurane to minimise suffering before decapitation.
Animals
Male mice, 6 weeks of age (C57BL/6J, Taconic) were randomly assigned to the experimental diets (Table 1) and housed individually. Mice were maintained on a 12 h light–dark cycle at 28 ± 1°C.
Diets
Feed provided as pellets were available ad libitum for 16 weeks. The diets contained the same amount (g/kg) of sucrose 50, cellulose 50, maize starch 100, mineral mix (American Institute of Nutrition (AIN) 93M MX) 47, vitamin mix (AIN 93 VX) 13, l-cysteine 3, choline bitartrate 2·5, and ethoxyquin 0·06. Salmon fillets were freeze-dried, ground and mixed with the other ingredients to make pellets. The salmon fillets were the sole protein source and provided 20 en% proteins, and 40 and 60 en% fat for FO- and SO-enriched salmon, respectively, due to higher fat content in the SO salmon fillets (Table 1). The fat contents of the salmon fillets were 26 g/100 g FO fillet and 33 g/100 g SO fillet. Additional vegetable oils were added to obtain a total lipid content of 35 en%. A mix of oils was used to preserve the fatty acid profile of the salmon fillets (FO and SO) regarding LA, α-linolenic acid (18 : 3n-3) and MUFA with reciprocal changes in SFA (Table 1). The full fatty acid profile of the salmon fillets and vegetable oils is given in Table S1 (available online). Feed intake was measured daily by weighing each food cup and spillage and subtracting the previously collected weight. Body weight was recorded once per week for all mice.
Endocannabinoids
Brain and liver were quickly snap-frozen in liquid N2. AEA and 2-AG were extracted and analysed by GC–MS/MS as described previously by Alvheim et al. (Reference Alvheim, Malde and Osei-Hyiaman10) in the mouse liver and cerebral cortex, and the salmon liver.
Phospholipid fatty acid profile
Liver, erythrocyte and adipose tissue lipids were extracted with chloroform–methanol (2:1, v/v) and PL were separated from neutral lipids by solid-phase extraction. Liver and epididymal white adipose tissue (eWAT) lipids were evaporated to dryness and recovered in chloroform to a concentration of 50 mg/ml lipids. An aliquot of 200 μl (10 mg lipids) was applied to a solid-phase extraction column (Isolute; Biotage). Erythrocyte lipids were evaporated to dryness and recovered with three washings of 100 ml chloroform and deposited to the solid-phase extraction column. Neutral lipids were eluted with 10 ml chloroform–methanol (98:2, v/v) and polar lipids were eluted with 20 ml methanol. The fatty acid composition in the phospholipid fraction of liver and erythrocytes, and the neutral fraction of adipose tissue were analysed by GC as described previously(Reference Lie and Lambertsen47, Reference Torstensen, Nanton and Olsvik48).
Blood chemistry
Blood was collected at the time of killing and separated into erythrocytes and plasma. Plasma hormone levels were determined using commercially available ELISA kits in accordance with the manufacturer's instructions for insulin (Ultrasensitive ELISA, mouse; DRG Instruments GmbH), leptin (Leptin (Mouse/Rat) ELISA; ALPCO Immunoassays) and adiponectin (Adiponectin (Mouse) Total, HMW ELISA; ALPCO Immunoassays).
Histology
Sections of eWAT and inguinal white adipose tissue (iWAT) were fixed in 4 % formaldehyde in 0·1 m-phosphate buffer for 24 h, washed in phosphate buffer, dehydrated in ethanol, and embedded in paraffin after clearing with xylene. Then, 5 μm-thick sections of the embedded tissue were stained with eosin and haematoxylin. The sections were visually examined using an Olympus BX 51 binocular microscope (Olympus) fitted with a Nikon DS-Fi1 camera (Digital Sight DS-Fi1; Nikon). Adipocyte size was measured using the interactive measurement module of an image analysis system equipped with an Olympus microscope (Olympus), a Nikon camera and NIS-elements software (Nikon). Thereafter, 200 adipocytes per tissue were randomly selected and measured by drawing a horizontal line between the cell membranes.
Immunohistochemistry
To distinguish macrophages from other cell types in the adipose tissue, the presence of F4/80, a transmembrane protein specific for macrophages, was visualised by immunohistochemistry. Samples were processed as described previously. In brief, 5 μm sections were deparafinised, rehydrated and endogenous peroxide was inactivated (3 % H2O2). To reduce non-specific staining, the sections were incubated in heat-inactivated normal goat serum (10 %, 10 min). The sections were then incubated overnight at 4°C with rat anti-mouse F4/80 (1:500; Serotec), subsequently washed in Tris-buffered saline (three times, 10 min) and incubated with horseradish peroxidase-conjugated rat anti-goat IgG (1:250; Serotec) for 2 h. After washing in Tris-buffered saline (three times, 10 min), specific binding of the antibody to macrophages was visualised using diaminobenzidine. The relative abundance of macrophages compared with fat cells was assessed by a scientist unaware of the experimental protocol.
Statistics
All data were analysed using STATISTICA (data analysis software system), version 9.0 (StatSoft, Inc.). Levene's test was used to test for homogeneity of variance. Data were analysed with Student's independent t test with a significance level of P< 0·05. Weekly body weight was measured by repeated-measures ANOVA and a statistical trend was set at P< 0·08. Data are presented as means with their standard errors. Samples of the liver and fillet of salmon were pooled – three to nine fish per tank in each of the three tanks to provide three fish from pooled samples. Mouse samples represent nine individual animals.
Results
Effect of the experimental diets in Atlantic salmon
Replacing dietary FO with SO resulted in a 19-fold increase in LA, significantly higher AA levels and nearly doubled the concentration of 2-AG (Fig. 1) in the salmon liver. There was no difference in final body weight, mean visceral somatic index ((visceral adipose tissue+internal organ)/body weight) or whole-fish proximate composition after 6 months of feeding (Table 2). However, fish fed the SO diet had significantly higher amounts of TAG and total lipid in the liver than fish fed the FO diet, with no difference in PL (Table 2). Feeding SO to Atlantic salmon increased fillet LA (530 %) and reduced EPA (71 %) and DHA (56 %) compared with fillets from Atlantic salmon fed FO (Table 3).

Fig. 1 Levels of the n-6 fatty acids (a) linoleic acid (LA) and (b) arachidonic acid (AA), and the endocannabinoids (c) 2-arachidonoylglycerol (AG) and (d) anandamide (AEA) in the liver of Atlantic salmon fed soyabean oil (SO, ![]() ) and fish oil (FO,
) and fish oil (FO, ![]() ). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of SO-fed salmon (P< 0·05).
). Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from those of SO-fed salmon (P< 0·05).
Table 2 Physical parameters in Atlantic salmon fed fish oil (FO) or soyabean oil (SO) (Mean values with their standard errors, n 3 from pooled samples)
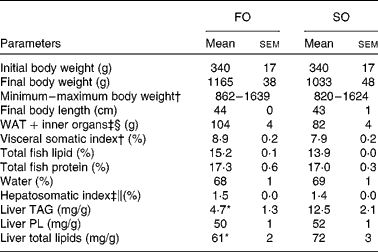
WAT, white adipose tissue; PL, phospholipids.
Mean value was significantly different from that of SO-fed salmon: * P< 0·04 (Student's independent t test).
† Pooled samples; nine fish per tank, three tanks.
‡ Pooled samples; six fish per tank, three tanks.
§ Includes intestine, stomach and spleen.
∥ Hepatosomatic index: (liver weight (g)/body weight (g)) × 100.
Table 3 n-6 and n-3 profile in total lipids of fillet and liver of Atlantic salmon fed fish oil (FO) and soyabean oil (SO) (Mean values with their standard errors, n 3 from pooled samples)
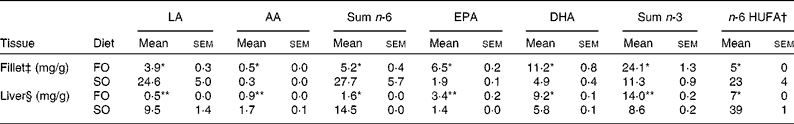
LA, linoleic acid; AA, arachidonic acid; HUFA, highly unsaturated fatty acids.
Mean values were significantly different from those of SO-fed salmon: * P< 0·04, ** P< 0·01.
† n-6 HUFA is calculated from the Lands equation(Reference Lands, Libelt and Morris4): (20 : 3n-6+20 : 4n-6+22 : 5n-6)/(20 : 3n-6+20 : 4n-6+22 : 5n-6+20 : 5n-3+22 : 5n-3+22 : 6n-3) × 100.
‡ Pooled samples; three fish from three tanks.
§ Pooled samples; six fish from three tanks; n 3.
Effect of the experimental diets in mice
Mice fed the SO salmon diet had higher amounts of LA and AA in the liver PL (Fig. 2(a) and (b)), erythrocyte-PL and neutral lipids of eWAT than mice fed the FO salmon diet (Table 4). Increasing dietary LA by replacing FO with SO in feed for Atlantic salmon significantly elevated liver 2-AG, elevated brain 2-AG and AEA, and significantly increased weight gain, feed efficiency and caused higher body weight (P< 0·08) in mice (Fig. 2). Mice fed the SO salmon diet had higher body weight than mice fed the FO salmon diet from week 6, but the trend was only significant from week 9 (P= 0·05–P< 0·07) with a significant difference in week 15 (P= 0·03). Energy intake and plasma concentrations of leptin, adiponectin and insulin did not differ between the dietary treatments (Table 5).
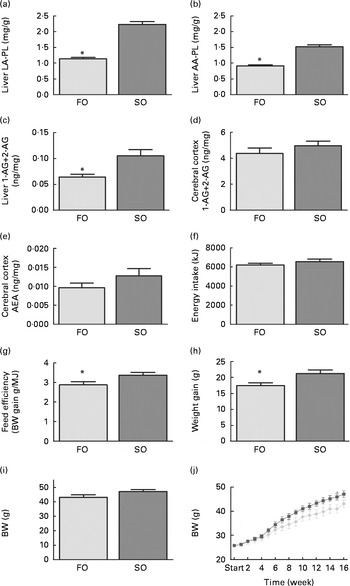
Fig. 2 Changes in (a) linoleic acid (LA), (b) arachidonic acid (AA) in liver phospholipids (PL), (c) liver 2-arachidonoylglycerol (AG), cerebral (d) 2-AG and (e) anandamide (AEA), and (f) energy intake, (g) feed efficiency, (h) weight gain, (i) final body weight and (j) weekly body weight in mice fed the fish oil (FO, ![]() ) and soyabean oil (SO,
) and soyabean oil (SO, ![]() ) salmon diets. Values are means, with their standard errors represented by vertical bars (n 8–9). * Mean values were significantly different from those of SO salmon-fed mice (P< 0·05). BW, body weight.
) salmon diets. Values are means, with their standard errors represented by vertical bars (n 8–9). * Mean values were significantly different from those of SO salmon-fed mice (P< 0·05). BW, body weight.
Table 4 n-6 and n-3 profile of erythrocyte and liver phospholipids, and neutral lipids of epididymal white adipose tissue (eWAT) in mice fed fish oil (FO) and soyabean oil (SO) salmon (Mean values with their standard errors, n 9)
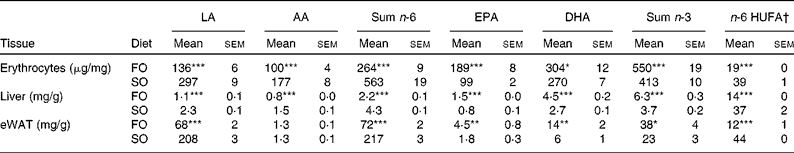
LA, linoleic acid; AA, arachidonic acid; HUFA, highly unsaturated fatty acids.
Mean values were significantly different from those of SO salmon-fed mice: * P< 0·03, ** P< 0·01, *** P< 0·0001.
† n-6 HUFA is calculated from the Lands equation(Reference Lands, Libelt and Morris4): (20 : 3n-6+20 : 4n-6+22 : 5n-6)/(20 : 3n-6+20 : 4n-6+22 : 5n-6+20 : 5n-3+22 : 5n-3+22 : 6n-3) × 100.
Table 5 Physical and biochemical parameters in mice fed fish oil (FO) or soyabean oil (SO) salmon†‡ (Mean values with their standard errors)
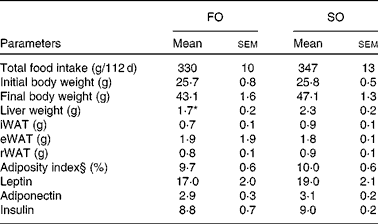
iWAT, inguinal white adipose tissue; eWAT, epididymal white adipose tissue; rWAT, retroperitoneal white adipose tissue.
* Mean value was significantly different from that of SO salmon-fed mice (P< 0·04).
† n 9 for food and energy intake, feed efficiency and body weight; n 6–7 for hormone levels.
‡ Feed efficiency: (body weight gain (g)/energy (MJ) intake).
§ Adiposity index: ((epididymal fat+inguinal fat+retroperitoneal fat)/eviscerated body weight) × 100.
Replacing FO with SO in feed for Atlantic salmon significantly lowered EPA and DHA in mice liver-PL, erythrocyte-PL and total lipids of eWAT, thereby decreasing the omega-3 index from 23 to 16 and increasing n-6 highly unsaturated fatty acids from 19 to 39 % (Table 4).
Adipose tissue accumulation and the adiposity index did not differ between mice fed the SO and FO salmon diets (Table 5). Staining eWAT and iWAT for the macrophage marker F4/80 showed considerably more F4/80-positive macrophages forming crown-like structures around adipocytes in mice fed the SO salmon diet than mice fed the FO salmon diet (Fig. 3). The SO salmon diet containing 8 en% LA resulted in larger adipocyte size in iWAT, but not in eWAT, than the FO salmon diet containing 1 en% LA (Fig. 3).
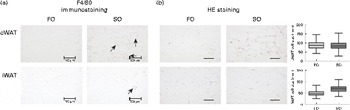
Fig. 3 Histology of epididymal white adipose tissue (eWAT) and inguinal white adipose tissue (iWAT) in mice fed the fish oil (FO) and soyabean oil (SO) salmon diets. (a) Immunostaining with the macrophage marker F4/80 in eWAT and iWAT of FO- and SO-fed mice. (b) Haematoxylin eosin (HE) staining and adipocyte size in eWAT and iWAT of mice fed the FO and SO salmon diets. Values are means, minimum and maximum ranges represented by vertical bars.
Discussion
Human consumption of soyabean oil has increased from 2·2 to 7·3 en% at the same time as the intake of EPA and DHA declined in the USA during the twentieth century(Reference Blasbalg, Hibbeln and Ramsden3). Increasing intake of LA has been linked to obesity in both humans(Reference Alvheim, Malde and Osei-Hyiaman10, Reference Massiera, Saint-Marc and Seydoux19, Reference Dayton, Hashimoto and Dixon21, Reference Ailhaud, Massiera and Weill51) and rodents(Reference Matias, Petrosino and Racioppi7, Reference Alvheim, Malde and Osei-Hyiaman10, Reference Madsen, Pedersen and Liaset18, Reference Massiera, Saint-Marc and Seydoux19, Reference Ikemoto, Takahashi and Tsunoda23, Reference Javadi, Everts and Hovenier52–Reference Takahashi and Ide54). In the present study, we found that mice fed the SO salmon diet with a high level of LA (8 en%) had higher liver concentrations of AA-PL and 2-AG. The elevated endocannabinoid activity in mice fed the SO salmon diet was associated with increased feed efficiency, higher weight gain and body weight (P< 0·8) compared with mice fed the FO salmon diet. We have previously shown that elevating dietary LA from 1 to 8 en% increases endocannabinoid production and induces obesity in mice fed high-fat diets, an effect that was reduced by adding 1 en% EPA and DHA to 8 en% LA diets(Reference Alvheim, Malde and Osei-Hyiaman10). The present and our previous findings are consistent with several reports that show how dietary fat alters endocannabinoid levels(Reference Artmann, Petersen and Hellgren5, Reference Wood, Williams and Pandarinathan6, Reference Berger, Crozier and Bisogno11–Reference Watanabe, Doshi and Hamazaki14, Reference Matias, Carta and Murru20, Reference Banni, Carta and Murru55).
The findings of lower weight gain and body weight in mice fed the FO salmon diet compared with mice fed the SO salmon diet are in line with the general notion that fish oil rich in EPA and DHA limits high-fat diet-induced obesity in rodents(Reference Belzung, Raclot and Groscolas26, Reference Hill, Peters and Lin27), an effect that is associated with reduced tissue levels of AA-PL(Reference Cunnane, Manku and Horrobin22). The diet based on SO-enriched salmon resulted in larger adipocyte size in iWAT and more macrophage infiltration in eWAT than the FO-fed salmon diet. Diets enriched with n-3 PUFA have been demonstrated to reduce adipose tissue inflammation in diet-induced obesity(Reference Huber, Loffler and Bilban56–Reference Todoric, Loffler and Huber58). The mechanism by which EPA and DHA reduce macrophage-induced adipose tissue inflammation has recently been demonstrated to be mediated by the stimulation of the fatty acid receptor GPR120(Reference Oh, Talukdar and Bae59). EPA and DHA can be converted to metabolic products such as resolvins and protectins with anti-inflammatory actions independent of the state of obesity(Reference Bannenberg, Chiang and Ariel60). Although both salmon diets contained relatively high amounts of EPA and DHA, the present data suggest that dietary LA of 8 en% decrease the anti-inflammatory properties of EPA and DHA. In keeping with the recent finding that sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue(Reference Ma, Liaset and Hao61), these data demonstrate that the background diet influences the anti-inflammatory properties of EPA and DHA.
Traditionally, farmed Atlantic salmon have consumed diets high in EPA and DHA and low in LA and α-linolenic acid. Introducing vegetable oils in farmed fish feed has altered the dietary fatty acid profile and fillet fatty acid composition(Reference Turchini, Torstensen and Ng45). Consistent with prior reports(Reference Torstensen, Bell and Rosenlund44–Reference Grisdale-Helland, Ruyter and Rosenlund46), replacing fish oil with soyabean oil in feed for Atlantic salmon increased dietary LA from 1·5 to 21 en% in feed, and altered the fatty acid profile of salmon fillet and liver by increasing LA and decreasing EPA and DHA after 6 months of feeding. To our knowledge, we are the first to show that dietary LA from soyabean oil elevated 2-AG in the salmon liver. Consistent with previous reports replacing fish oil with soyabean oil(Reference Ruyter, Moya-Falcon and Rosenlund62) or a blend of vegetable oils(Reference Torstensen, Espe and Stubhaug63–Reference Morais, Pratoomyot and Torstensen65), we found that Atlantic salmon fed soyabean oil accumulated significantly more TAG and total lipids in the liver than salmon fed fish oil. Liver is the main source of de novo fatty acid synthesis, and the activation of liver cannabinoid receptor 1 stimulates de novo fatty acid synthesis in mice by increasing the activity of the transcription factor sterol regulatory element binding protein-1c (SREBP-1c), and its target enzymes fatty acid synthase and acetyl-CoA carboxylase(Reference Osei-Hyiaman, DePetrillo and Pacher8). Recent studies have demonstrated that replacing fish oil with vegetable oil in Atlantic salmon up-regulated the expression of SREBP-1c and fatty acid synthase in the salmon liver(Reference Morais, Pratoomyot and Taggart66), affecting pathways of cholesterol and lipoprotein metabolism(Reference Morais, Pratoomyot and Torstensen65). It is therefore likely that an elevated endocannabinoid tone in the liver of salmon fed soyabean oil may have stimulated the hepatic de novo synthesis of fatty acids. In Atlantic salmon, replacing fishmeal and fish oil with high levels of plant protein (80 %) and a vegetable oil blend (70 %) of rapeseed, palm and linseed oil increased whole-body lipids, the visceral somatic index and liver lipid stores after 12 months of feeding(Reference Torstensen, Espe and Stubhaug63) concomitant with a significant lower body weight(Reference Torstensen, Espe and Sanden67). In another study, replacing fish oil with a vegetable oil blend (rapeseed, palm and linseed oil) increased body weight in Atlantic salmon receiving vegetable oil compared with fish oil possibly due to the long-term effects increasing nutrient utilisation(Reference Torstensen, Bell and Rosenlund44). We did not find any effect on body weight or the visceral somatic index in salmon when soyabean oil replaced fish oil for 6 months, indicating interacting effects between oil source and protein source, or length of feeding required to affect body weights or visceral fat stores in Atlantic salmon(Reference Torstensen, Bell and Rosenlund44, Reference Torstensen, Espe and Stubhaug63).
There have been increasing concerns about the decreasing content of EPA and DHA in farmed Atlantic salmon. Norwegian surveillance data(68) report a moderate increase in LA levels in the fillets of farmed Atlantic salmon from 1·1 g/100 g in 2005 to 1·6 g/100 g in 2010 and a decrease in EPA+DHA from 2·7 g/100 g to 2·1 g/100 g in the same period. In the present study, Atlantic salmon was fed soyabean oil, a major dietary source of LA, resulting in 2·5 g LA and 0·7 g EPA+DHA/100 g fillet, representing an extreme model where vegetable oils replace fish oil in feed for Atlantic salmon. Although recognised as suitable alternatives to fish oil in feed for Atlantic salmon, the present findings of elevated liver endocannabinoid and lipid accumulation in salmon fed soyabean oil suggest that future fish oil replacement in farmed Atlantic salmon should pay attention to the choice of vegetable oils with regard to LA content. However, although lower than the FO salmon diet, mice fed the SO salmon diet for 16 weeks had a high omega-3 index and low n-6 highly unsaturated fatty acids (16 and 39 %, respectively), indicating that farmed Atlantic salmon fed vegetable oil remain a significant dietary source of EPA and DHA.
In summary, replacing fish oil with soyabean oil in feed for Atlantic salmon introduces high dietary levels of LA in Atlantic salmon and elevates AA, endocannabinoid activity and TAG accumulation in the salmon liver. Mice consuming Atlantic salmon fed soyabean oil have higher liver levels of AA-PL and 2-AG, higher feed efficiency, higher weight gain and more adipose tissue inflammation than mice fed the FO salmon diet. Thus, lower dietary levels of LA may improve metabolic functions associated with obesity.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512003364
Acknowledgements
The present study was supported by the National Institute of Nutrition and Seafood Research, Bergen, Norway, the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, USA, and the Research Council of Norway 186908/l10. The authors' responsibilities were as follows: A. R. A. and M. K. M. designed the study and conducted the mouse trial. A. R. A., Y. H. L., E.-J. L. and H. H. L. analysed the data. A. R. A. performed the statistical analysis and had primary responsibility for the final content; B. E. T. designed the study and conducted the fish trial. A. R. A., L. M. and B. E. T. wrote the paper; J. R. H. contributed to the design and provided the essential equipment. The authors declare that they have no conflict of interest.










