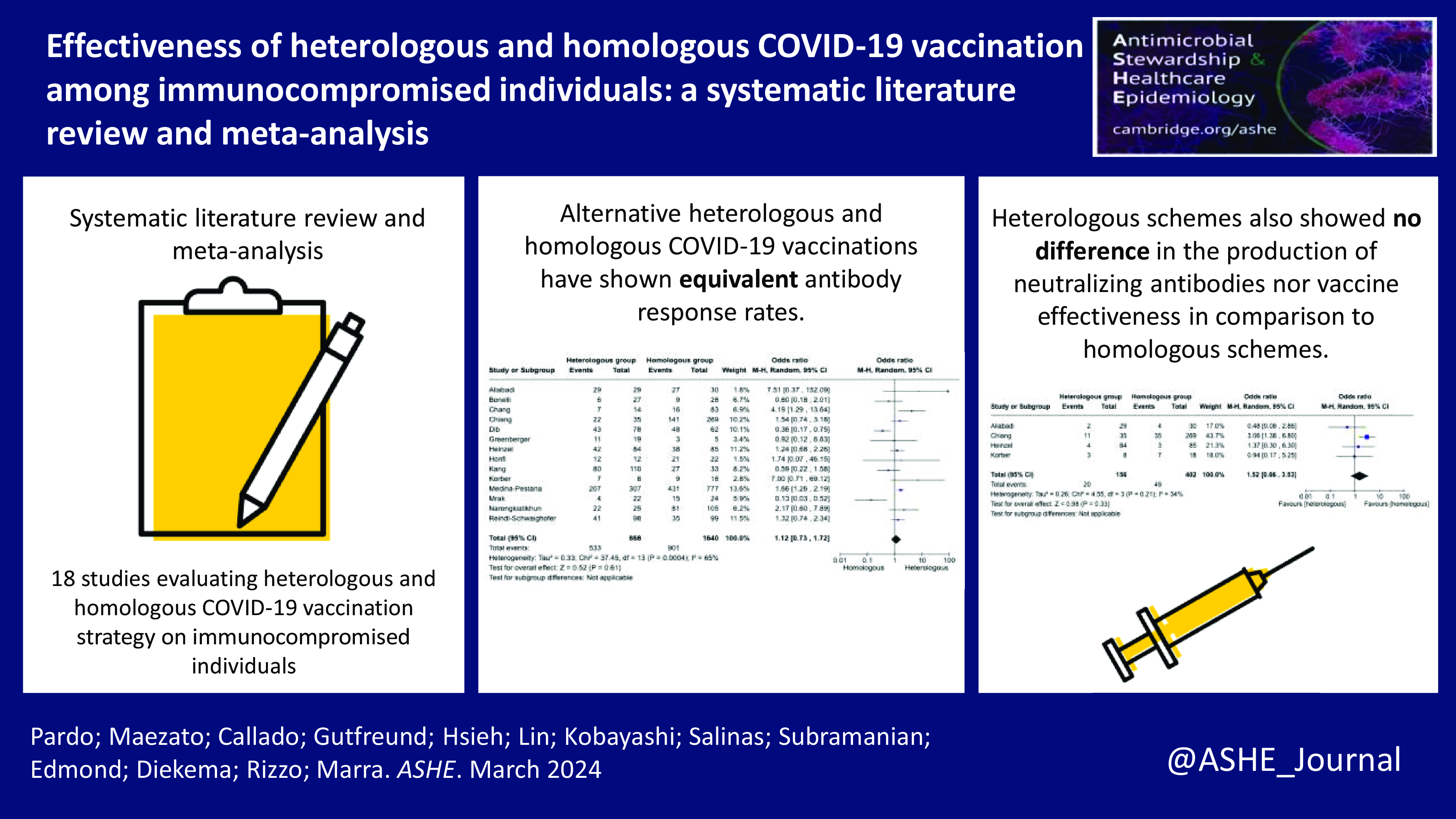
Background
The COVID-19 pandemic emerged as a severe public health issue. 1 SARS-CoV-2 infected 774 million people and caused 7 million deaths worldwide as of January 2024. Reference Mathieu, Ritchie and Rodés-Guirao2 The first vaccine against this disease was authorized by the US Food and Drug Administration (FDA) on December 11, 2020. Reference Polack, Thomas and Kitchin3 Several studies have evaluated vaccine efficacy in healthy individuals or those with stable chronic medical conditions. Reference Polack, Thomas and Kitchin3 However, immunocompromised individuals were excluded from trials during the early stages of the pandemic, leading to a lack of data on vaccine efficacy for this group. Reference Marra, Kobayashi and Suzuki4 Recently, some investigations suggested that vaccine effectiveness and humoral immune response in immunocompromised individuals were lower than in immunocompetent people. Reference Goldman, Robinson, Uldrick and Ljungman5,Reference Mair, Berger and Berghoff6 Given higher COVID-19 complication and mortality rates among those immunocompromised, Reference Fung and Babik7 it is important to quantify vaccine effectiveness (VE) in this group and propose strategies to enhance immune response.
Currently, with new variants and evidence of reduced immunity induced by COVID-19 vaccines, booster doses are being administrated. Reference Feikin, Higdon and Abu-Raddad8 However, obtaining boosters of the same type of COVID-19 vaccine sometimes poses a challenge due to inadequate access to mRNA vaccines in low- and middle-income countries, the rollout of Janssen or AstraZeneca vaccines followed by subsequent shortages of the primary vaccine types, or nationwide shifts to mRNA vaccines. Therefore, heterologous vaccination, where vaccines with different vectors or delivery systems from those used in the initial doses are administered as boosters, is employed. This approach has been adopted in many countries, enhancing vaccination flexibility and reducing vaccine inequity. Reference Tatar, Shoorekchali, Faraji, Seyyedkolaee, Pagán and Wilson9 Additionally, heterologous strategies may provide immunologic advantages to extend the breadth and longevity of protection. Reference Atmar, Lyke and Deming10 Therefore, studying the effectiveness of heterologous approaches is crucial for informing public health measures, particularly in countries where a diverse array of vaccines is not readily accessible.
We aimed to evaluate the effectiveness of heterologous vaccination on immunocompromised individuals through COVID-19 outcomes (levels of anti-SARS-CoV-2 spike protein IgG, neutralizing antibodies, symptomatic COVID-19, hospitalization, and death) in comparison to homologous approaches.
Methods
Systematic literature review and inclusion and exclusion criteria
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement Reference Moher, Liberati, Tetzlaff and Altman11 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. Reference Stroup12 This study was registered on Prospero (https://www.crd.york.ac.uk/PROSPERO/) on July 3, 2023 (registration number CRD42023440193). Institutional Review Board approval was not required. Immunocompromised individuals were defined as those treated with immunosuppressive medication (eg, corticosteroids, chemotherapy, or other immunosuppressive medications), chronic renal failure under hemodialysis, autoimmune and inflammatory rheumatic and musculoskeletal disease, solid organ transplant, hematopoietic stem cell transplant, HIV, or active cancer (current cancer, in treatment, or received diagnosis within last 12 months). Reference Pilishvili, Fleming-Dutra and Farrar13,Reference Martinson and Lapham14 Heterologous vaccination strategies were defined as schemes in which the booster dose has different vectors or delivery systems from the ones used in the primary series. Homologous strategies were defined as three doses of the same vaccine, with the same vectors and delivery systems (Figure 1). We only included those who got at least one booster. One Janssen dose is equivalent to the primary series with two doses of other COVID-19 vaccines. The review included manuscripts published from January 1, 2020 to September 29, 2023. There were no language restrictions. Inclusion criteria for studies in this systematic literature review were as follows: original research manuscripts; published in peer-reviewed, scientific journals; conducted in acute care settings that evaluated the effectiveness of heterologous versus homologous COVID-19 vaccines in immunocompromised individuals with randomized clinical trial design; and observational study design. Commentaries, studies with overlapping individuals, studies in pediatric populations, and studies in preprint were excluded. Studies in which there was no comparison between heterologous and homologous vaccination and evaluating less than 3 doses were excluded.

Figure 1. Examples of heterologous and homologous vaccination schemes. Note: One Janssen dose is equivalent to the primary series with two doses of other COVID-19 vaccines.
Search strategy
We performed literature searches in PubMed, Cumulative Index to Nursing and Allied Health (CINAHL), Embase (Elsevier Platform), Cochrane Central Register of Controlled Trials, Scopus, and Web of Science. The entire search strategy is described in Supplementary Appendix 1. We reviewed the reference lists of retrieved articles to identify studies that were not identified from the preliminary literature searches. To filter the 16,252 articles obtained from the databases, titles and/or abstracts were assessed by two investigators (I.P and A.M.M.) to exclude articles using the inclusion criteria. All disparities were resolved through consensus.
Data abstraction and quality assessment
Of six independent reviewers (I.P, A.R.M, G.Y.C, M.K.H, M.C.G, and V.L), two independently abstracted data for each article using a standardized abstraction form (Supplemental Form 1). Reviewers resolved disagreements by consensus. All reviewers recorded data on study design, publication year and calendar time, population selection, setting, analyzed vaccines, serological response definition, and side effects associated with vaccination. Our primary outcome was positive antibody response according to the cut-off presented by the analyzed study.
Secondary outcomes were to evaluate the vaccine effectiveness through the number of symptomatic SARS-CoV-2 infections after COVID-19 vaccines, as well as the number of hospitalizations, and deaths related to COVID-19. The risk of bias was assessed using the Downs and Black scale. Reference Downs and Black15 Reviewers answered all original questions except for question #27, which was modified to a yes or no. The highest possible score achievable on this scale was 28. Two authors performed the scale independently, and discrepancies were solved by consensus.
Patient consent statement
The present investigation is a systematic literature review and meta-analysis of published data, so no patient informed consent was required.
Statistical analysis
For the meta-analysis, we compared positive antibody responses between heterologous versus homologous vaccinations. We weighted each study for the analysis using the approach outlined by DerSimonian and Laird. 16 We performed stratified analyses of the associations between anti-SARS-CoV-2 spike protein IgG production in different types of immunocompromised states, in studies that evaluated neutralizing antibodies, in studies with symptomatic COVID-19 after receiving the COVID-19 vaccines, and in studies classified as good per the Downs and Black score. We did not include studies that did not report the absolute number of individuals that produced anti-SARS-CoV-2 spike protein IgG after the third vaccine dose in our stratified analysis. We assessed heterogeneity between studies using both the I 2 statistic and the Cochran Q statistic test. We analyzed with the Cochrane Review Manager (RevMan) Web edition 4.12.0. To examine publication bias, we visually inspected a funnel plot using RevMan (Supplemental Figure 1A and 1B) and also evaluated by applying the Egger test with Comprehensive Meta-Analysis version 4 software (Englewood, NJ).
Results
Characteristics of included studies in the systematic literature review
Eighteen studies met the inclusion criteria Reference Sharifi Aliabadi, Karami and Barkhordar17–Reference Thompson, Ngecu and Stoddart34 and were included in the systematic literature review (Figure 2 and Table 1). Five were randomized clinical trials, Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Bonelli, Mrak and Tobudic18,Reference Heinzel, Schrezenmeier and Regele26,Reference Mrak, Sieghart and Simader31,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33 four were retrospective cohort studies, Reference Chang, Chiang and Kim19,Reference Chiang, Alejo and Mitchell20,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25,Reference Thompson, Ngecu and Stoddart34 and nine were prospective cohort studies. Reference Dib, Le Corre and Ortiz21–Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Honfi, Gémes and Szabó27–Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Narongkiatikhun, Noppakun and Chaiwarith32 Most studies were conducted in Austria (four studies), Reference Bonelli, Mrak and Tobudic18,Reference Heinzel, Schrezenmeier and Regele26,Reference Mrak, Sieghart and Simader31,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33 and in the United Stated of America (four studies), Reference Chang, Chiang and Kim19,Reference Chiang, Alejo and Mitchell20,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25,Reference Thompson, Ngecu and Stoddart34 followed by Chile (two studies), Reference Dib, Le Corre and Ortiz21,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24 and Iran (one study), Reference Sharifi Aliabadi, Karami and Barkhordar17 Turkey (one study), Reference Erol, Kuloğlu and Kayaaslan22 United Kingdom (one study), Reference Fendler, Shepherd and Au23 Hungary (one study), Reference Fendler, Shepherd and Au23 Korea (one study), Reference Kang, Lee and Huh28 Germany (one study), Reference Körber, Holzmann-Littig and Wilkens29 Brazil (one study), Reference Medina-Pestana, Almeida Viana and Nakamura30 and Taiwan (one study). Reference Narongkiatikhun, Noppakun and Chaiwarith32 Studies were performed between March 2021 and January 2023, Reference Sharifi Aliabadi, Karami and Barkhordar17–Reference Thompson, Ngecu and Stoddart34 varying from 1 month to 10 months.

Figure 2. Literature search for articles on COVID-19 vaccine effectiveness among immunocompromised individuals.
Table 1. Summary of characteristics of studies included in the systematic literature review

Abbreviations: AU/mL Arbitrary Units per milliliter, AZ, AstraZeneca; BAU/mL, Binding Antibody Units per milliliter; D&B, Downs and Black; HSCT, Hematopoietic Stem Cell Transplantation; IQR, Interquartile Range; KTR, Kidney transplant recipients; Nab, Neutralizing Antibodies; NR, Not Reported; RCT, Randomized Clinical Trial; SD, Standard Deviation; SOT, Solid Organ Transplant; SV, CoronaVac; UK, United Kingdom.
*GMC = geometric mean concentration.
In our qualitative analysis, eighteen studies including 3,019 individuals evaluated the effect of a heterologous vaccination strategy on immunocompromised individuals using outcomes related to COVID-19 (levels of anti-SARS-CoV-2 spike protein IgG, neutralizing antibodies, COVID-19, hospitalization, and death) in comparison to homologous strategies. Of the eighteen studies evaluated, sixteen evaluated Pfizer/BioNTech mRNA COVID-19 vaccine. Reference Bonelli, Mrak and Tobudic18–Reference Mrak, Sieghart and Simader31,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 Nine of these studies also evaluated Moderna mRNA COVID-19 vaccine, Reference Bonelli, Mrak and Tobudic18–Reference Chiang, Alejo and Mitchell20,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25,Reference Kang, Lee and Huh28,Reference Körber, Holzmann-Littig and Wilkens29,Reference Mrak, Sieghart and Simader31,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 seven studies also assessed AstraZeneca COVID-19 vaccine, Reference Bonelli, Mrak and Tobudic18,Reference Fendler, Shepherd and Au23,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25,Reference Honfi, Gémes and Szabó27–Reference Körber, Holzmann-Littig and Wilkens29,Reference Mrak, Sieghart and Simader31 five studies analyzed Janssen COVID-19 vaccine, Reference Chiang, Alejo and Mitchell20,Reference Heinzel, Schrezenmeier and Regele26,Reference Kang, Lee and Huh28,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 five studies assessed CoronaVac COVID-19 vaccine, Reference Dib, Le Corre and Ortiz21,Reference Erol, Kuloğlu and Kayaaslan22,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Narongkiatikhun, Noppakun and Chaiwarith32 and one study evaluated Sputinik COVID-19 vaccine. Reference Honfi, Gémes and Szabó27 There were two additional studies: one study compared the PastoCovac (also called Soberana 02, manufactured in the Pasteur Institute of Iran in collaboration with the Finlay Vaccine Institute of Cuba) Reference Sharifi Aliabadi, Karami and Barkhordar17 and CoronaVac COVID-19 vaccines. Reference Narongkiatikhun, Noppakun and Chaiwarith32 Twelve studies evaluated transplant recipients, Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Chang, Chiang and Kim19–Reference Erol, Kuloğlu and Kayaaslan22,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Heinzel, Schrezenmeier and Regele26,Reference Kang, Lee and Huh28–Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 being ten studies of solid organ transplants, Reference Chang, Chiang and Kim19–Reference Dib, Le Corre and Ortiz21,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Heinzel, Schrezenmeier and Regele26,Reference Kang, Lee and Huh28–Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 one study of hematopoietic stem cell transplant, Reference Sharifi Aliabadi, Karami and Barkhordar17 and one study evaluated both types of transplants (solid and hematopoietic stem cell transplant). Reference Erol, Kuloğlu and Kayaaslan22 Also, two studies evaluated individuals under immunosuppressive therapy, Reference Bonelli, Mrak and Tobudic18,Reference Mrak, Sieghart and Simader31 one study reviewed individuals with autoimmune and inflammatory rheumatic and musculoskeletal disease, Reference Honfi, Gémes and Szabó27 two studies investigated individuals with malignancies, Reference Fendler, Shepherd and Au23,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25 and one study analyzed patients undergoing maintenance hemodialysis. Reference Narongkiatikhun, Noppakun and Chaiwarith32
Studies showed significant variations in the reporting of serological test characteristics. There was limited consensus on the time of performance after the third dose, cutoff levels for antibody positivity, and the specific type of serological test conducted (Supplemental Table 1). Among the eighteen studies included in the systematic literature review, one study did not provide information on when the serological test was performed. Reference Fendler, Shepherd and Au23 Additionally, nine studies did not report any investigation into cellular immunity, Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Chang, Chiang and Kim19,Reference Chiang, Alejo and Mitchell20,Reference Erol, Kuloğlu and Kayaaslan22,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25,Reference Kang, Lee and Huh28,Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Narongkiatikhun, Noppakun and Chaiwarith32 while the remaining nine studies that conducted this analysis employed different approaches to assess cellular immunity. Reference Bonelli, Mrak and Tobudic18,Reference Dib, Le Corre and Ortiz21,Reference Fendler, Shepherd and Au23,Reference Heinzel, Schrezenmeier and Regele26,Reference Honfi, Gémes and Szabó27,Reference Körber, Holzmann-Littig and Wilkens29,Reference Mrak, Sieghart and Simader31,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 (Supplemental Table 1).
Regarding the quality assessment scores, ten studies were considered good (>18 of 28 possible points) per the Downs and Black quality tool, Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Bonelli, Mrak and Tobudic18,Reference Dib, Le Corre and Ortiz21,Reference Fendler, Shepherd and Au23,Reference Heinzel, Schrezenmeier and Regele26,Reference Körber, Holzmann-Littig and Wilkens29–Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33 seven studies were considered fair (15–18 points), Reference Chang, Chiang and Kim19,Reference Chiang, Alejo and Mitchell20,Reference Erol, Kuloğlu and Kayaaslan22,Reference Gaete-Argel, Saavedra-Alarcón and Sauré24,Reference Honfi, Gémes and Szabó27,Reference Kang, Lee and Huh28,Reference Thompson, Ngecu and Stoddart34 and one study was considered poor quality (<14 points). Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25
Outcomes measures
Overall, fourteen studies, Reference Sharifi Aliabadi, Karami and Barkhordar17–Reference Dib, Le Corre and Ortiz21,Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25–Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33 including 2,508 immunocompromised individuals evaluated the antibody response (anti-SARS-CoV-2 spike protein IgG) and were included in the meta-analysis. The positive antibody response rate in 2,508 immunocompromised individuals ranged from 18% to 100%. In total, 61.4% of individuals had positive antibody response in the heterologous vaccination group, while 54.9% had positive antibody response in the homologous vaccination group. The heterologous vaccination group had no difference in the odds of developing anti-SARS-CoV-2 spike protein IgG compared to the homologous vaccination (pooled odds ratio 1.12 [95% Cl: 0.73–1.72] (Figure 3). From the fourteen studies included in the meta-analysis, eight studies Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Chang, Chiang and Kim19,Reference Dib, Le Corre and Ortiz21,Reference Honfi, Gémes and Szabó27–Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Narongkiatikhun, Noppakun and Chaiwarith32 also analyzed anti-SARS-Cov-2 spike protein IgG neutralizing antibodies. In the stratified analysis, the pooled odds ratio for developing neutralizing antibodies among the heterologous group was 1.48 [95% Cl: 0.72–3.05] compared to homologous strategies (Supplemental Table 2). Regarding the heterologous vaccination response among different immunocompromising conditions, 100% of hematological transplant recipients Reference Sharifi Aliabadi, Karami and Barkhordar17 and autoimmune and inflammatory rheumatic and musculoskeletal individuals, Reference Honfi, Gémes and Szabó27 88% of individuals undergoing maintenance hemodialysis, Reference Narongkiatikhun, Noppakun and Chaiwarith32 62.6% of solid organ transplant recipients, Reference Chang, Chiang and Kim19–Reference Dib, Le Corre and Ortiz21,Reference Kang, Lee and Huh28–Reference Medina-Pestana, Almeida Viana and Nakamura30,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33 57.9% of individuals with malignant tumor, Reference Greenberger, Saltzman, Senefeld, Johnson, DeGennaro and Nichols25 and 20.4% of individuals on immunosuppressive therapy Reference Bonelli, Mrak and Tobudic18,Reference Heinzel, Schrezenmeier and Regele26 had a positive antibody response (Supplemental Table 2).

Figure 3. Forest plot of COVID-19 vaccine response (anti-SARS-CoV-2 spike protein IgG) after three doses of COVID-19 vaccine [n = 14 studies] with heterologous and homologous vaccination schemes. Odds ratios (OR) were determined with the Mantel–Haenszel random-effects method. Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel.
Four studies, Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Chiang, Alejo and Mitchell20,Reference Heinzel, Schrezenmeier and Regele26,Reference Körber, Holzmann-Littig and Wilkens29 with a total of 558 immunocompromised individuals, also evaluated symptomatic COVID-19 (Figure 4). In a group of 156 individuals in the heterologous vaccination, 12.8% developed COVID-19, while in the homologous vaccination group of 402 individuals, 12.2% developed COVID-19. The pooled odds ratio to acquire COVID-19 in the heterologous vaccination group was 1.52 [95% CI: 0.66–3.53] compared to the homologous strategy (Supplemental Table 2).

Figure 4. Forest plot of COVID-19 after three doses of COVID-19 vaccine [n = 4 studies] with heterologous and homologous vaccination schemes. Odds ratios (OR) were determined with the Mantel–Haenszel random-effects method. Abbreviations: CI, confidence interval; M-H, Mantel–Haenszel.
Among eighteen studies, three Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Heinzel, Schrezenmeier and Regele26,Reference Medina-Pestana, Almeida Viana and Nakamura30 reported other COVID-19 outcomes, including hospitalizations and deaths related to COVID-19. In total, hospitalizations were seen in 0.30% (4/1,314) of patients, including 0.71% (3/422) in the heterologous strategy and 0.11% (1/892) in the homologous strategy. Deaths were observed in 0.76% (10/1,314) of patients, including 0.95% (4/422) in the heterologous strategy and 0.67% (6/892) in the homologous strategy among the three studies Reference Sharifi Aliabadi, Karami and Barkhordar17,Reference Heinzel, Schrezenmeier and Regele26,Reference Medina-Pestana, Almeida Viana and Nakamura30
The results of meta-analyses represented substantial heterogeneity for studies evaluating anti-SARS-Cov-2 spike protein IgG in individuals who received the COVID-19 vaccine heterologous or homologous scheme (heterogeneity P = 0.61, I2 = 65%), and homogenous for studies evaluating VE on COVID-19 in individuals who received the COVID-19 vaccine heterologous or homologous scheme (heterogeneity P = 0.21, I2 = 34%), respectively.
Publication bias
We conducted a publication bias analysis through funnel plot visualization of studies evaluating COVID-19 vaccine response with anti-SARS-CoV-2 spike protein IgG and studies evaluating COVID-19 (Supplemental Figure 1A and 1B). In both graphs, the studies were reasonably balanced around the pooled ORs with little evidence of publication bias. The Egger’s test also did not indicate publication bias among those included studies in the meta-analysis (P = 0.55).
Discussion
This systematic literature review and meta-analysis demonstrate that heterologous COVID-19 strategies result in comparable antibody responses to homologous strategies among immunocompromised patients. Moreover, effectiveness was found to be similar between those with heterologous and homologous booster strategies. Despite moderate heterogeneities, these findings support the flexibility of using vaccines with different vectors and delivery systems, considering supply and logistical factors.
As new variants of COVID-19 continue to emerge, the COVID-19 vaccination program aims to prevent severe disease through the administration of new booster shots. For example, the JN.1 lineage became predominant in United States in January 2024, Reference Link-Gelles, Ciesla and Mak35 The World Health Organization (WHO) has classified this variant as a Variant of Interest (VOI) due to its rapidly increasing spread. 36 The emergence of this VOI and other variants, which can spread easily even among individuals who have had a previous infection or vaccination, raises the risk of reinfection or breakthrough cases. This is particularly concerning for people with weakened immune systems, as the virus can persist for longer periods, increasing the likelihood of generating new variants that may be more challenging to manage. Reference Truong, Ryutov and Pandey37,Reference Baang, Smith and Mirabelli38 Additionally, the prevalence of immunosuppressed individuals has increased from 2.7% in 2013 to 6.3% in 2021, further highlighting the urgency of addressing the unique challenges faced by this population. Reference Martinson and Lapham14 There is a pressing need for studies assessing the effectiveness of vaccines against new variants specifically in the immunosuppressed population. In the meta-analysis, all studies measured the vaccine response using anti-SARS-CoV-2 spike protein IgG. However, a limited number of studies evaluated the neutralizing ability against the virus, directly measuring the capacity to inhibit viral replication. Further research is essential in the immunosuppressed population to distinguish between antibody production and actual protection against symptomatic COVID-19. Additionally, it is crucial to investigate the durability of this protection. Moreover, there is a need for specific strategies tailored to individuals with severe immunosuppression compared to those with milder degrees of immunosuppression. Having target recommendations for different levels of immunosuppression would enhance the precision and effectiveness of our guidance.
The demonstrated similarity in antibody response and effectiveness between heterologous and homologous vaccination strategies highlights the adaptability and potential efficacy of diverse vaccine regimens. Although the CDC allows adults to receive a different manufacturer booster from the type of the primary series, children aged less than 4 years are still recommended to receive homologous boosters. 39 A number of low- and middle-income countries still do not have adequate accessibility of COVID-19 vaccines and have high unmet demand. Reference Yamey, Garcia and Hassan40,Reference Fox, Choi and Lin41 Adopting heterologous booster strategies could be valuable for reinforcing the immune response in immunocompromised individuals amid emerging COVID-19 variants. Tailoring vaccination approaches for this vulnerable population is crucial, and our study provides empirical support for considering alternative schedules. These insights can guide the development of evidence-based recommendations, assisting policymakers in more effectively allocating resources and optimizing vaccine distribution. Our study advocates for ongoing vigilance in the face of evolving variants, emphasizing the need for continuous monitoring and adaptive public measures for sustained protection of immunocompromised individuals against severe outcomes of COVID-19.
This study has several limitations. First, most studies included were non-randomized (13 of 18), which introduces potential sources of bias in our findings. Non-randomized designs may be influenced by confounding variables, limiting the ability to establish causal relationships with confidence. This aspect underscores the need for caution in drawing definitive conclusions about the comparative effectiveness of heterologous and homologous vaccination. Furthermore, the diverse array of serological tests adopted across the studies, each with different cutoff levels for antibody positivity, poses a significant challenge. This heterogeneity could introduce variability in the interpretation of antibody response rates. Secondly, the study’s focus on the measurement of vaccine response primarily through anti-SARS-CoV-2 spike protein IgG, while informative, may not provide a comprehensive understanding of the overall immune response. The exclusive reliance on IgG levels, without a thorough examination of other antibody types or neutralizing capacity, limits the depth of our insights into the true protective efficacy of the vaccines. We agree with the FDA guidance cautioning against using antibody testing as a sole indicator of immunity, emphasizing the need for a more nuanced understanding of the relationship between serological markers and protection against symptomatic COVID-19. As such, this study calls for future research to delve into these complexities and broaden the scope of assessment for a more holistic evaluation of vaccine effectiveness in immunocompromised individuals. Thirdly, we lack substantial data on the vaccine’s effectiveness in preventing severe disease or mortality among immunocompromised populations. Among all the studies reviewed, only four reported symptomatic COVID-19, while three outlined other outcomes (hospitalization and death). There was no data on these four included studies about the unvaccinated individuals for vaccine effectiveness. Fourth, we considered the Janssen dose as equivalent to two doses of other vaccines. In four studies, Reference Chiang, Alejo and Mitchell20,Reference Heinzel, Schrezenmeier and Regele26,Reference Reindl-Schwaighofer, Heinzel and Mayrdorfer33,Reference Thompson, Ngecu and Stoddart34 the Janssen booster dose was evaluated after two doses of mRNA vaccines, which would total four doses in our classification. However, this situation accounts for approximately only 1% of all patients included. Hence, it did not have a big influence on our results focusing on three COVID-19 doses. Fifth, given the immunocompromised nature of the study population, addressing the potential impact of underlying comorbidities on vaccine response and effectiveness might be relevant. Certain comorbidity conditions could influence the outcomes and should be acknowledged as potential confounders. Lastly, our systematic literature review did not specifically address the comparison of different COVID-19 variants or the role of repeated infections, which could potentially contribute to variations in infection and mortality rates. This review tries to contribute to the broader discussion on equitable access to effective vaccination, particularly for vulnerable populations.
In conclusion, heterologous COVID-19 vaccines have demonstrated comparable rates of antibody response and effectiveness compared to homologous strategies in immunocompromised individuals. This approach could potentially help address global disparities in vaccine distribution. More studies are necessary to evaluate vaccine effectiveness for different vaccination strategies, VE against new variants, and the clinical significance of anti-SARS-CoV-2 spike protein IgG antibody levels in immunosuppressed populations, the most vulnerable to severe COVID-19 disease.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ash.2024.369.
Acknowledgments
We thank Kioko Kusuki de Oliveira, librarian from Instituto Israelita de Ensino e Pesquisa of Hospital Israelita Albert Einstein, for her crucial assistance in defining our research strategy.
Financial support
This study was not funded.
Competing interests
Authors have no commercial or financial involvement, except for AS, whose institution receives research funding from Moderna, Inc., Pfizer, Inc., and Janssen, Inc, and DJD, whose institution has research contracts and received payments for the development of antifungal susceptibility testing technology from BioMerieux, Inc., and Affinity Biosensors, Inc., and received payments for consulting regarding an investigational vaccine from ExBaq, Inc.