First-principles calculations were employed to investigate the adsorption-induced surface stress of self-assembled alkanethiolate monolayers on a 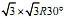 Au(111) surface as a function of the alkyl chain length. A recently developed fully nonlocal van der Waals density functional was used to accurately account for the chain-chain interactions. We found that surface charge redistribution produces compressive surface stress, while chain-chain interactions produce tensile surface stress. The stress induced by surface charge redistribution is about one order of magnitude greater than that of chain-chain interactions. We observed that the chain-chain interactions play an important role in determining the molecular configuration during adsorptions, and also contribute significantly to the induced anisotropic tensile (positive) surface stress. As the chain length increases the tensile stress increases at a rate of ∼ 0.32 (∼ 0.18) N/m for the direction perpendicular (parallel) to the chain tilt direction.
Au(111) surface as a function of the alkyl chain length. A recently developed fully nonlocal van der Waals density functional was used to accurately account for the chain-chain interactions. We found that surface charge redistribution produces compressive surface stress, while chain-chain interactions produce tensile surface stress. The stress induced by surface charge redistribution is about one order of magnitude greater than that of chain-chain interactions. We observed that the chain-chain interactions play an important role in determining the molecular configuration during adsorptions, and also contribute significantly to the induced anisotropic tensile (positive) surface stress. As the chain length increases the tensile stress increases at a rate of ∼ 0.32 (∼ 0.18) N/m for the direction perpendicular (parallel) to the chain tilt direction.
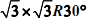 AU(111) Surface: a van der Waals Density Functional Study
AU(111) Surface: a van der Waals Density Functional Study

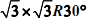 AU(111) Surface: a van der Waals Density Functional Study
AU(111) Surface: a van der Waals Density Functional Study
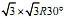 Au(111) surface as a function of the alkyl chain length. A recently developed fully nonlocal van der Waals density functional was used to accurately account for the chain-chain interactions. We found that surface charge redistribution produces compressive surface stress, while chain-chain interactions produce tensile surface stress. The stress induced by surface charge redistribution is about one order of magnitude greater than that of chain-chain interactions. We observed that the chain-chain interactions play an important role in determining the molecular configuration during adsorptions, and also contribute significantly to the induced anisotropic tensile (positive) surface stress. As the chain length increases the tensile stress increases at a rate of ∼ 0.32 (∼ 0.18) N/m for the direction perpendicular (parallel) to the chain tilt direction.
Au(111) surface as a function of the alkyl chain length. A recently developed fully nonlocal van der Waals density functional was used to accurately account for the chain-chain interactions. We found that surface charge redistribution produces compressive surface stress, while chain-chain interactions produce tensile surface stress. The stress induced by surface charge redistribution is about one order of magnitude greater than that of chain-chain interactions. We observed that the chain-chain interactions play an important role in determining the molecular configuration during adsorptions, and also contribute significantly to the induced anisotropic tensile (positive) surface stress. As the chain length increases the tensile stress increases at a rate of ∼ 0.32 (∼ 0.18) N/m for the direction perpendicular (parallel) to the chain tilt direction.