INTRODUCTION
Pneumonia and diarrhoea are the leading causes of child mortality [Reference Liu1]. In 2011, two million children died before reaching their fifth birthday because of pneumonia and diarrhoea worldwide [Reference Walker2]. Although a declining trend in mortality from pneumonia and diarrhoea has been witnessed in some industrialized countries, pneumonia and diarrhoea are still an important source of morbidity in these regions [Reference Podewils3]. For example, in the Western Pacific region, the total episodes of pneumonia and diarrhoea in children aged <5 years were 256·3 million and 12·2 million, respectively, in 2010 [Reference Walker2].
Pneumonia and diarrhoea are largely preventable, and hence it is essential to identify the risk factors and take targeted preventive measures [Reference Bhutta4]. Existing studies have confirmed some individual-level biological factors for pneumonia and diarrhoea, such as being underweight, stunting and zinc deficiency [Reference Walker2]. Some of these poverty-related risk factors, such as sub-optimum breastfeeding, under-nutrition and zinc deficiency, are shared by pneumonia and diarrhoea, and these overlapping risk factors may result in the geographical co-distribution of these diseases [Reference Walker5].
Prior studies also have reported that climate may play an important role in the transmission of pneumonia and diarrhoea, highlighting that high temperature [Reference Checkley6, Reference Green7] and rainfall [Reference Garcia-Vidal8, Reference Hashizume9] may trigger pneumonia and diarrhoea. As projected by an Intergovernmental Panel on Climate Change, the Earth's surface average temperature will increase, and there will be more intense rainy seasons in Asia, Africa, and the Pacific [10]. As climate change continues, the burden of pneumonia and diarrhoea in these regions may increase, although there are still regional differences and contrasting effects of climate on pneumonia and diarrhoea due to different aetiological agents.
Australia shoulders a considerable burden of childhood pneumonia and diarrhoea [Reference Scallan11, Reference Rudan12]. It is urgently required that the spatio-temporal patterns of childhood pneumonia and diarrhoea in Australia are revealed. This study explored the spatio-temporal patterns, geographical co-distribution and socio-ecological determinants of childhood pneumonia and diarrhoea in Queensland, Australia.
MATERIALS AND METHODS
Data collection
Queensland is located in the northeast of Australia. Its mean summer temperature is 25°C and mean winter temperature is 15°C. There is significant variation in mean annual rainfall across Queensland, varying from <150 mm/year in the southwest to 4000 mm/year in the northern coast [13]. Data on emergency department visits (EDVs) by postcode from 1 January 2007 to 31 December 2011 in Queensland were obtained from Queensland Health. The anonymized EDV data were classified according to the International Classification of Diseases, 10th version (ICD-10). In this study, we included EDVs with the principal cause coded as pneumonia (ICD-10 codes: J12–J18) and diarrhoeal disease of any cause (ICD-10 codes: A00–A03, A04, A05, A06·0–A06·3, A06·9, A07·0–A07·2, A07·9, A08–A09) among children aged 0–14 years. Ethical approval was obtained from the Human Research Ethics Committee of Queensland University of Technology (Australia) prior to data collection. Patient information was de-identified and thus no written informed consent was required. Data on weather (including temperature and rainfall) were supplied by the world climate website, including interpolated monthly mean temperature and monthly rainfall. Temperature and rainfall values for each postal area during the study period were extracted using the ArcMap software package (ESRI Inc., USA). Data for the same period for each postcode on the socioeconomic index for areas (SEIFA) and population size, were obtained from the Australian Bureau of Statistics [13]. SEIFA is a product developed by the Australian Bureau of Statistics that ranks areas in Australia according to relative socioeconomic advantage and disadvantage. Lower SEIFA values indicate lower socioeconomic status.
Statistical analysis
We plotted the decomposed daily distributions of EDVs for childhood pneumonia and diarrhoea using a time-series approach. The change in EDVs for childhood pneumonia and diarrhoea from 2008–2009 to 2010–2011 was calculated using the following equation:
where M c represents the morbidity change, EDV i2010–2011 represents the EDVs for childhood pneumonia (diarrhoea) for postal area i during 2010–2011, EDV i2008–2009 represents the EDVs for childhood pneumonia (diarrhoea) for postal area i during 2008–2009, and population i refers to the population for postal area i.
A Bayesian conditional autoregressive (CAR) model [Reference Besag, York and Mollié14] was used to estimate the relative risk of EDVs for childhood pneumonia and diarrhoea in postcode districts with a range of independent variables, including temperature, rainfall and SEIFA.
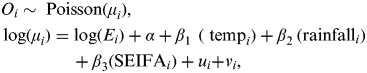 $$\eqalign{& O_i \sim \; {\rm Poisson}(\mu _i )\comma \cr & \log (\mu _i ) = \log (E_i ) + \alpha + \beta _{{\rm 1}\; \;} (\; {\rm temp}_i ) + \beta _{{\rm 2}\;} ({\rm rainfall}_i ) \cr & \hskip41 + \beta _{\rm 3} ({\rm SEIFA}_i ) + u_i {\rm +} v_i\comma } $$
$$\eqalign{& O_i \sim \; {\rm Poisson}(\mu _i )\comma \cr & \log (\mu _i ) = \log (E_i ) + \alpha + \beta _{{\rm 1}\; \;} (\; {\rm temp}_i ) + \beta _{{\rm 2}\;} ({\rm rainfall}_i ) \cr & \hskip41 + \beta _{\rm 3} ({\rm SEIFA}_i ) + u_i {\rm +} v_i\comma } $$
where O i is the observed counts of pneumonia (diarrhoea) from the ith postcode (i = 1, …, 424), E i , the expected number of cases in postal area i, is an offset to control for population, α is the intercept, β 1 is the coefficient for temperature, β 2 is the coefficient for rainfall, β 3 is the coefficient for SEIFA, u i is a spatially structured random effect with mean zero and variance σ u 2, and v i is a spatially unstructured random effect with mean zero and variance σ v 2.
We conducted an initial burn-in of 10 000 iterations that were subsequently discarded. Convergence was evaluated through visual inspection of posterior density plots, history plots, and autocorrelation of selected parameters, and it was reached within the first 10 000 iterations for the model. We conducted a subsequent set of 200 000 iterations for the accuracy. Model selection was conducted by comparing the deviance information criterion (DIC) of different models. In this study, we defined May–October as the dry season, and January, February, March, April, November and December as the wet season.
Time-series analysis was conducted using the R statistical environment, version 2.15.3 (R Foundation, Austria). Visual maps were created using ArcGIS version 9.3 (ESRI Inc.). Spatial cluster analysis was conducted using SatScan v. 9.1 (http://satscan.software.informer.com/9.1/), and the Bayesian CAR model was conducted using WinBugs software, version 1.4.3 (MRC Biostatistics Unit, 2008).
RESULTS
Summary statistics
Table 1 presents the summary statistics of EDVs for childhood pneumonia and diarrhoea, mean temperature, rainfall and SEIFA by postcode in Queensland. The average counts of childhood pneumonia and diarrhoea were 43·7 and 138·5, respectively, and the mean values of mean temperature, rainfall and SEIFA were 20·1°C, 95·7 mm, and 976·6.
Table 1. Summary statistics for emergency department visits for childhood pneumonia and diarrhoea, monthly mean temperature and rainfall, and SEIFA by postcode in Queensland, Australia, during 2007–2011

SEIFA, Socioeconomic index for areas.
Spatial pattern
Figure 1 shows the spatial distribution of rates of EDVs for childhood pneumonia and diarrhoea, illustrating that EDVs for pneumonia were the highest in the central west, northwest and far north of Queensland, and the EDVs for childhood diarrhoea were the highest in the northwest of Queensland (Mount Isa city). Figure 2 illustrates the change in EDVs for childhood pneumonia and diarrhoea from years 2008–2009 to 2010–2011, indicating that EDVs for pneumonia and diarrhoea changed from the northwest or southeast of Queensland during the past few years.

Fig. 1. The spatial distribution of emergency department visits for (a) childhood pneumonia and (b) diarrhoea in Queensland, from 2007 to 2011.

Fig. 2. The change of emergency department visits for (a) childhood pneumonia and (b) diarrhoea in Queensland, from 2008–2009 to 2010–2011.
Temporal pattern
Figure 3 shows the decomposed daily distributions of EDVs for childhood pneumonia and diarrhoea, showing a distinct seasonal trend for the two diseases, especially for pneumonia. This figure indicates that EDVs for childhood pneumonia in Queensland were more likely to occur in the cold season. The particularly great number of EDVs for childhood pneumonia in 2009 is because of the 2009 pandemic H1N1 influenza.

Fig. 3. The daily distribution of emergency department visits for (a) childhood pneumonia and (b) diarrhoea in Queensland, from 2007 to 2011.
Geographical co-distribution
The cluster results in Figure 4 reveal that EDVs for childhood pneumonia and diarrhoea in Queensland were co-distributed in Mount Isa.

Fig. 4. The spatial clusters of emergency department visits for (a) childhood pneumonia and (b) diarrhoea in Queensland, from 2007 to 2011. RR, Relative risk.
Socio-ecological drivers
The effect of socio-ecological factors on EDVs for childhood pneumonia and diarrhoea in the dry and wet seasons is reflected in Table 2. SEIFA played an important role in driving the distribution of pneumonia, highlighting that more EDVs for pneumonia occurred in regions with low socioeconomic status. The relationship between rainfall and EDVs for pneumonia was significant in wet seasons, with a 3% [95% confidence interval (CI) 1–5] increase in EDVs for pneumonia for each 10-mm increase in monthly average rainfall. Rainfall was also significantly associated with EDVs for diarrhoea in both dry and wet seasons, with a 4% (95% CI 2–7) increase in EDVs for diarrhoea for each 10-mm increase in monthly average rainfall. Mean temperature was negatively associated with EDVs for diarrhoea in wet seasons, but not in dry seasons.
Table 2. Bayesian spatial conditional autoregressive models of socio-ecological drivers of childhood pneumonia and diarrhoea in Queensland, Australia

RR, Relative risk; CI, confidence interval; SEIFA, socioeconomic index for areas.
* P < 0.05.
Posterior estimated relative risks of childhood pneumonia and diarrhoea reveal that high-risk areas of childhood pneumonia were located in the northwest of Queensland, and high-risk areas of childhood diarrhoea were located in central west Queensland (Fig. 5). Estimated residual variation after taking into account the socio-ecological factors indicate that high-incidence postal areas for childhood pneumonia were located in the far north and northwest of Queensland, and high-incidence postal clusters for childhood diarrhoea were located in Mount Isa (Fig. 6).

Fig. 5. Relative risk (RR) of (a) childhood pneumonia and (b) diarrhoea from the spatial conditional autoregressive model.

Fig. 6. Spatial random effects for emergency department visits for (a) childhood pneumonia and (b) diarrhoea.
DISCUSSION
This study has yielded several notable findings. There was a strong seasonal trend in EDVs for childhood pneumonia, with more cases occurring in the cold season. Children suffering pneumonia and diarrhoea who visited emergency departments in Queensland from 2007 to 2011 were mainly from the central west, northwest and far north areas of Queensland. According to the cluster analysis results, Mount Isa is the high-risk area for both childhood pneumonia and diarrhoea. Interestingly, in recent years, Mount Isa has experienced a substantial decrease in EDVs for childhood pneumonia and diarrhoea, with EDVs for these diseases moving from the west to southeast of Queensland. We found SEIFA played a relatively more important role than climate in the driving the spatial transmission of childhood pneumonia, while climate may be more essential in the spread of childhood diarrhoea. Only in wet seasons was rainfall significantly associated with EDVs for childhood pneumonia. Low temperature may significantly increase EDVs for childhood diarrhoea, also only in wet seasons.
Mount Isa city, a major lead, zinc and copper producer, is the largest emitter of sulphur dioxide, lead and some other metals in Australia [15]. It has been convincingly documented that the blood lead levels of children in Mount Isa, especially those aged 1–4 years, is much higher than in children from other regions of Australia [16], and the consequent lifelong negative health and intellectual impacts of lead exposure on children have also been extensively reported [Reference Lanphear17, Reference Tong18]. In this study, we found that pneumonia and diarrhoea in children were co-distributed in Mount Isa, highlighting that there might be some common risk factors in this area. Exposure to air pollutants (e.g. sulphur dioxide) emitted by mining could increase hospital admissions for childhood pneumonia [Reference Barnett19]. Mining also had a significant adverse effect on the semi-arid freshwater aquatic system in Mount Isa [Reference Taylor20]. The densities of bacterial indicators in remnant pools throughout Leichhardt River have exceeded acceptable guidelines, which might expose children to greater risk of diarrhoea. In this study, we also found the risk areas for childhood pneumonia and diarrhoea changed from the northwest to southeast of Queensland, and the EDVs for childhood pneumonia and diarrhoea in Mount Isa have decreased sharply (although still high) in recent years, indicating that protective measures may have been taken to safeguard children from the continuously adverse impacts of mining.
In this study, we found the average SEIFA score was significantly associated with childhood pneumonia, but not diarrhoea, implying that socioeconomic factors may play a more prominent role in pneumonia than diarrhoea in Queensland. This finding conflicts with previous study conducted in China which found socioeconomic factors played a more important part in driving the transmission of pneumonia than diarrhoea [Reference Feng21]. This inconsistency reveals that the patterns of risk factors for pneumonia and diarrhoea in developed and developing countries may not be the same, suggesting that future preventive measures should focus on the economic characteristics of specific regions. With regards to the mechanism of social and economic impacts on childhood pneumonia, we found in the literature that most risk factors for childhood pneumonia (e.g. being underweight) are socioeconomically related [Reference Walker2]. Children in the lower socioeconomic groups appear to be living in more crowded houses and suffer under-nutrition more often than those with higher socioeconomic status, possibly increasing their risk of getting pneumonia.
High rainfall was found to be significantly associated with more pneumonia and diarrhoea, especially in wet seasons. Two studies, so far, have formally explored the relationship between rainfall and childhood pneumonia in the Philippines [Reference Paynter22] and the USA [Reference Ebi23], both using a time-series approach, but did not find significant results. On rainy days, children are more likely to spend time indoors, which may increase crowding and their exposure to biomass fuel smoke, and decrease their sunlight exposure, possibly resulting in a higher risk of getting pneumonia. The association between high rainfall and more EDVs for childhood diarrhoea found in our study corresponds to the findings of previous studies in Brazil [Reference Andrade24] and the USA [Reference Drayna25]. Increased rainfall may increase the risk of sewage overflow which leads to water supply contamination [Reference Curriero26]. Further, runoff of human excreta in soil and subsurface may increase, and result in more concentrations of pathogens in surface water. Turbulences may be caused by increased heavy rainfall, leading to sediment re-suspension and dispersing accumulated pathogens. Apart from high rainfall, low temperature was also found to be associated with more diarrhoeal cases in wet seasons in the present study, while little evidence on the relationship between temperature and pneumonia was found. Rotavirus has been reported as the most important cause of acute and severe childhood diarrhoea in industrialized countries [Reference Malek27–Reference Parashar29], and low temperature increases the survival and replication of rotavirus [Reference D'Souza, Hall and Becker30].
This study has several strengths. To the best of our knowledge, this is the first study to explore the geographical co-distribution of childhood pneumonia and diarrhoea. An advanced Bayesian spatial model was used to quantify the effect of socio-ecological factors on both childhood pneumonia and diarrhoea. The results from this study, especially the high-risk areas of pneumonia and diarrhoea we identified, may have important implications for future control and prevention of these diseases in Queensland. Two major limitations should also be acknowledged. First, the disease data we collected from emergency departments may underestimate the actual infected population because only children with severe symptoms would visit emergency departments for treatment. Second, only aggregated data were used, which may result in some measurement bias.
CONCLUSIONS
Childhood pneumonia and diarrhoea were predominantly distributed in the northwest of Queensland, and Mount Isa was the region where these two childhood diseases co-distributed. In recent years, the high-risk areas of these diseases has changed from the northwest to southeast of Queensland. Low temperature and high rainfall were associated with more childhood diarrhoea cases, and low SEIFA score was associated with more childhood pneumonia cases. Future precautionary measures should be taken before the rainy seasons to prevent children from the impact of pneumonia and diarrhoea.
ACKNOWLEDGEMENTS
Z.X. was funded by a China Scholarship Council Postgraduate Scholarship and Queensland University of Technology fee waiving scholarship; S.T. was supported by a National Health and Medical Research Council Research Fellowship (no. 553043).
DECLARATION OF INTEREST
None.











