Article contents
Role of nanoparticle interaction in magnetic heating
Published online by Cambridge University Press: 21 June 2019
Abstract
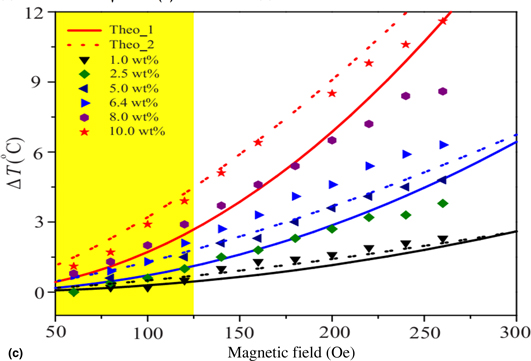
Magnetic nanoparticles have many potential applications in therapeutics and drug delivery. Heating by magnetic nanoparticles for hyperthermia application has gained tremendous popularity as a non-invasive treatment for tumor ablation. The heating effect of magnetic nanoparticles at different concentrations (1–10 wt%) in the fluid is investigated by varying the alternating magnetic field (60–260 Oe). The observed temperature rise (ΔT) shows an unusual increase with applied field in a higher nanoparticle concentration. In contrast to the previous model, the present study shows that temperature rise is more rapid in the higher particle concentration (~10 wt%) and low applied field (<125 Oe), and ΔT varies as H3/2 instead of H2.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 4
- Cited by


