No CrossRef data available.
Article contents
Enhanced lithium-ion transport in organosilyl electrolytes for lithium-ion battery applications
Published online by Cambridge University Press: 30 September 2019
Abstract
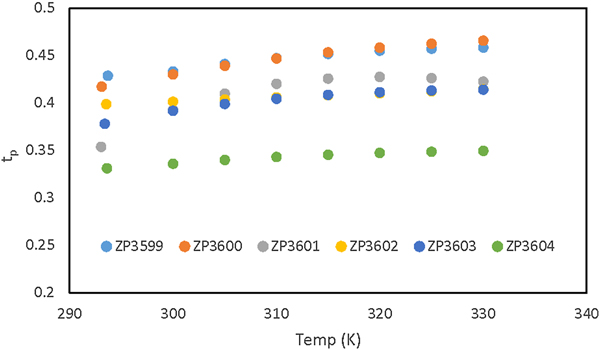
The authors report on 7Li, 19F, and 1H pulsed field gradient NMR measurements of 26 organosilyl nitrile solvent-based electrolytes of either lithium bis(trifluorosulfonyl)imide (LiTFSI) or lithium hexafluorophosphate. Lithium transport numbers (as high as 0.50) were measured and are highest in the LiTFSI electrolytes. The authors also report on solvent blend electrolytes of fluoroorganosilyl (FOS) nitrile solvent mixed with ethylene carbonate (EC) and diethyl carbonate. Solvent diffusion measurements on an electrolyte with 6% FOS suggest both the FOS and EC solvate the lithium cation. By comparing lithium transport and transference numbers, the authors find less ion pairing in FOS nitrile carbonate blend electrolytes and difluoroorganosilyl nitrile electrolytes.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019


