No CrossRef data available.
Article contents
Ultrasonic-induced morphological change of micro/nanodeposits and current change in electrochemical migration
Published online by Cambridge University Press: 27 September 2019
Abstract
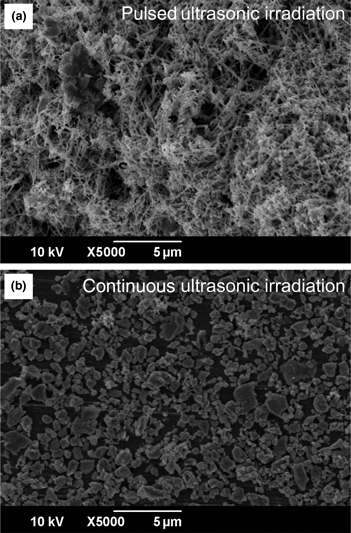
To examine the influence of ultrasonic irradiation on electrochemical migration (ECM), the morphology of micro/nanodeposits and current change were studied. The morphology of deposits synthesized by ECM varied with the types of ultrasonic irradiation: continuous or pulsed irradiation generates only particles or deposits composed of wires, dendrites, and particles. The measured ECM current change over time concludes that both mechanical and sonochemical effects contributed to the morphological change of deposits. Shock waves by cavitation mechanically formed the fragmented deposits and the sonochemical effect decreases the ionic concentration corresponding to decreasing current, inhibiting the formation of wires and dendritic deposits.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019



