Article contents
Polydiacetylene ribbons formed using the controlled evaporative self-assembly (CESA) method
Published online by Cambridge University Press: 12 October 2018
Abstract
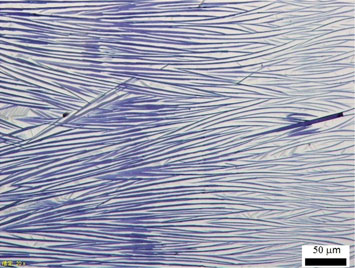
Methods for the control of molecular deposition and orientation are critical for the development of organic electronic devices. Here, we show the fabrication of ribbons of the optical material polydiacetylene (PDA) using a controlled evaporative self-assembly method. The ability to form these ribbons is highly dependent on both the side groups on the PDA as well as the solvent used in the preparation. Arrays of ribbons of one type of PDA, poly[1,6-di(N-carbazolyl)-2,4-hexadiyne], with widths on the order of 1–2 µm and lengths of 100s of micrometers, could be successfully obtained with good orientation.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 1
- Cited by


